

Synthesis of γ-Fe2O3 Nanocubes Decorated Graphene/CdS Nanocomposites with Enhanced Photocatalytic Performance
Received date: 2017-05-21
Online published: 2017-10-10
Supported by
Project supported by the National Natural Science Foundation of China (Nos. 51272094, 51602129) and the Natural Science Foundation of Jiangsu Province (Nos. BK20171295, BK20150507).
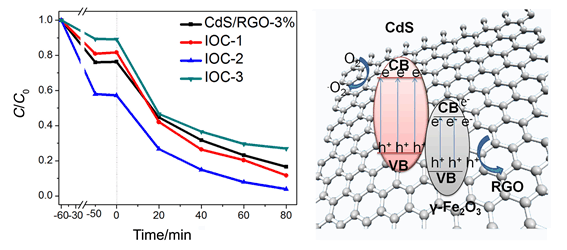
With Prussian blue (PB) as the precursor for γ-Fe2O3, the tri-component CdS/RGO/γ-Fe2O3 photocatalyst was prepared through loading PB nanocubes and CdS nanoparticles on graphene oxide (GO) nanosheets, followed by a calcination process in inert atmosphere (N2). The content of γ-Fe2O3 in the CdS/RGO/γ-Fe2O3 photocatalyst can be adjusted by changing the loading amount of PB, and the cubic morphology of PB was maintained after the calcination. The composition, structure, morphology and light absorption of the as-prepared products were investigated by X-ray diffraction (XRD), X-ray energy dispersive spectroscopy (EDS), field emission scanning electron microscopy (SEM), transmission electron microscopy (TEM), infrared spectroscopy (FT-IR), Raman spectroscopy and ultraviolet-visible (UV-vis) spectroscopy. The photocatalytic activity of the ternary photocatalysts was evaluated by the degradation of the organic pollutant of Rhodamine B (RhB) under visible-light irradiation. It was found that the degradation process of RhB follows pseudo-first-order kinetics. Compared to the bi-component CdS/RGO photocatalyst, the tri-component CdS/RGO/γ-Fe2O3 exhibited greatly enhanced photocatalytic activity, demonstrating that the γ-Fe2O3 played an important role in the photocatalytic process. The CdS/RGO/γ-Fe2O3 composite with PB loading amount of 12 mg exhibits the highest photocatalytic degradation efficiency of about 99.8% and the highest apparent reaction rate constant (k) value of about 0.03289 min-1, which is almost 2.9 times and 1.8 times higher than that of CdS and CdS/RGO, respectively. This result indicates that a suitable loading amount of γ-Fe2O3 is important to optimize the photocatalytic performance of the CdS/RGO/γ-Fe2O3 composites. Moreover, owing to the ferromagnetism of γ-Fe2O3, the CdS/RGO/γ-Fe2O3 photocatalyst could be easily separated from the reaction solution for recycling by a magnet. A possible photocatalytic mechanism was also proposed based on the photoluminescence (PL) characterization and the active species capture experiment. It was demonstrated that the enhanced photocatalytic degradation properties of CdS/RGO/γ-Fe2O3 composites can be ascribed to the excellent conductivity of RGO and the construction of Z-scheme heterostructure between CdS and γ-Fe2O3, which facilitate the transport and separation of photogenerated carriers.
Key words: γ-Fe2O3; graphene; CdS; photocatalysis; mechanism
Wu Jiajia , Ji Zhenyuan , Shen Xiaoping , Miao Xuli , Xu Keqiang . Synthesis of γ-Fe2O3 Nanocubes Decorated Graphene/CdS Nanocomposites with Enhanced Photocatalytic Performance[J]. Acta Chimica Sinica, 2017 , 75(12) : 1207 -1214 . DOI: 10.6023/A17050220
[1] Aarthi, T.; Narahari, P.; Madras, G. J. Hazard. Mater. 2007, 149, 725.
[2] Gu, S. H.; Wang, L. Z.; Zhang, J. L. Chin. J. Chem. 2017, 35, 153.
[3] Higashimoto, S.; Hikita, K.; Azuma, M. Chin. J. Chem. 2017, 35, 165.
[4] Wang, J. T.; Xiao, C.; Wu, X. Y. Chin. J. Chem. 2017, 35, 189.
[5] Li, X. D.; Zhang, Q. H.; Wang, H. Z. Chin. J. Chem. 2017, 35, 196.
[6] Qin, H. X.; Bian, Y. Y.; Zhang, Y. X. Chin. J. Chem. 2017, 35, 203.
[7] Cui, S. Z.; Yang, H. P.; Sun, H. H. Acta Chim. Sinica 2016, 74, 995. (崔素珍, 杨汉培, 孙慧华, 聂坤, 吴俊明, 化学学报, 2016, 74, 995.)
[8] Carey, J. H.; Lawrence, J.; Tosine, H. M. B. Environ. Contam. Tox. 1976, 16, 697.
[9] Wang, E. J.; Yang, H. Y.; Cao, Y. A. Acta Chim. Sinica 2009, 67, 2759(in Chinese). (王恩君, 杨辉云, 曹亚安, 化学学报, 2009, 67, 2759.)
[10] Bae, E.; Choi, W. Environ. Sci. Technol 2003, 37, 147.
[11] Wang, Y. W.; Zhu, Y. H.; Yang, X. L. Chin. J. Chem. 2017, 35, 949.
[12] Chang, J.; Zhang, W. J.; Hong, C. Y. Chin. J. Chem. 2017, 35, 1016.
[13] Jiang, L. P.; Wang, S. J.; Shi, L. Y. Chin. J. Chem. 2017, 35, 183.
[14] Cheng, J. S.; Wang, W. H.; Zhu, W. J. Chin. J. Chem. 2016, 34, 53.
[15] Wang, D. B.; Zhao, L. X.; Guo, L. H.; Zhang, H.; Wan, B.; Yang, Y. Acta Chim. Sinica 2015, 73, 388(in Chinese). (王大彬, 赵利霞, 郭良宏, 张辉, 万斌, 杨郁, 化学学报, 2015, 73, 388.)
[16] Bi, F.; Muhammad, F.; Liu, W. Chin. J. Chem. 2015, 33, 112.
[17] Abe, R.; Takata, T.; Sugihara, H. Chem. Commun. 2005, 30, 3829.
[18] Higashi, M.; Abe, R.; Teramura, K. Chem. Phys. Lett. 2008, 452, 120.
[19] Li, C. Q.; Luo, L. T.; Xiong, G. W. Acta Chim. Sinica 2010, 68, 1023(in Chinese). (李长全, 罗来涛, 熊光伟, 化学学报, 2010, 68, 1023.)
[20] Ba-Abbad, M. M.; Kadhum, A. A. H.; Mohamad, A. B. Int. J. Therm. Environ. Eng. 2010, 1, 37.
[21] Xie, Y. P.; Yu, Z. B.; Liu, G.; Ma, X. L.; Cheng, H. M. Energy. Environ. Sci. 2014, 7, 1895.
[22] Zhang, N.; Zhang, Y.; Pan, X.; Yang, M. Q.; Xu, Y. J. J. Phys. Chem. C, 2012, 116, 18023.
[23] Ye, X. J.; Dai, X.; Meng, S. G. Chin. J. Chem. 2017, 35, 217.
[24] Kashiath, L.; Namratha, K.; Byrappa, K. J. Alloy. Compd. 2016, 695, 799.
[25] Lee, J.; Kim, Y.; Kim, J. K.; Kim, S.; Min, D.; Jang, D. Appl. Catal. B 2017, 205, 433.
[26] Khan, S.; Han, J. S.; Lee, S. Y. Chin. J. Chem. 2017, 35, 159.
[27] Cong, R. M.; Luo, Y. J.; Yu, H. Q. Acta Chim. Sinica 2012, 68, 1971(in Chinese). (丛日敏, 罗运军, 于怀清, 化学学报, 2012, 68, 1971.)
[28] Li, H. J.; Zhou, Y.; Chen, L.; Luo, W. J.; Xu, Q. F.; Wang, X. Y.; Xiao, M.; Zou, Z. G. Nanoscale 2013, 5, 11933.
[29] Vaquero, F.; Navarro, R. M.; Fierro, J. L. G. Appl. Catal. B 2017, 753.
[30] Li, Q.; Guo, B. D.; Yu, J. G.; Ran, J. R.; Zhang, B. H.; Yan, H. J.; Gong, J. R. J. Am. Chem. Soc. 2011, 133, 10878.
[31] Li, Y. G.; Wei, X. L.; Li, H. J.; Wang, R. R.; Feng, J.; Yun, H.; Zhou, A. N. RSC Adv. 2015, 5, 14704.
[32] Liu, X. J.; Pan, L. K.; Lv, T.; Zhu, G.; Sun, Z.; Sun, C. Q. Chem. Commun. 2011, 47, 11984.
[33] Zhang, Y. H.; Tang, Z. R.; Fu, X. Z.; Xu, Y. J. ACS Nano 2010, 4, 7303.
[34] Zhang, N.; Yang, M. Q.; Liu, S. Q.; Sun, Y. G.; Xu, Y. J. Chem. Rev. 2015, 115, 10307.
[35] Quan, Q.; Lin, X.; Zhang, N.; Xu, Y. J. Nanoscale 2017, 9, 2398.
[36] Han, C.; Zhang, N.; Xu, Y. J. Nano Today 2016, 11, 351.
[37] Yuan, L.; Yang, M. Q.; Xu, Y. J. Nanoscale 2014, 6, 6335.
[38] Liu, Y.; Zhou, L.; Hu, Y.; Guo, C. F.; Qian, H. S.; Zhang, F. M.; Lou, X. W. J. Mater. Chem. 2011, 21, 18359.
[39] Li, N.; Zhang, J.; Tian, Y.; Zhao, J. H.; Zuo, W. Chem. Eng. J. 2017, 308, 377.
[40] Chen, Y.; Liu, K. R. J. Alloy. Compd. 2017, 697, 161.
[41] Jia, X. H.; Dai, R. R.; Lian, D. D.; Han, S.; Wu, X. Y.; Song, H. J. Appl. Surf. Sci. 2017, 392, 268.
[42] Wang, L.; Wei, H. W.; Fan, Y. J.; Gu, X.; Zhan, J. H. J. Phys. Chem. C 2009, 113, 14119.
[43] Liu, Y.; Yu, L.; Hu, Y.; Guo, C. F.; Zhang, F. M.; Lou, X. W. Nanoscale 2012, 4, 183.
[44] Zhang, L.; Wu, H. B.; Madhavi, S.,; Hng, H. H.; Lou, X. W. J. Am. Chem. Soc. 2012, 134, 17388.
[45] He, H.; Klinowski, J.; Forster, M. Chem. Phys. Lett. 1998, 287, 53.
[46] Singh, A. P.; Mishra, M.; Sambyal, P.; Gupta, B. K.; Singh, A.; Dhawan, S. K. J. Mater. Chem. A 2014, 2, 3581.
[47] Meng, N. N.; Zhou, Y. F.; Nie, W. Y.; Chen, P. P. J. Nanopart. Res. 2016, 18, 241.
[48] Kudin, K. N.; Ozbas, B.; Schniepp, H. C. Nano Lett. 2008, 8, 36.
[49] Sirivisoot, S.; Harrison, B. S. Int. J. Nanomedicine 2015, 10, 4447.
[50] Xu, J.; Wang, L.; Cao, X. J. Chem. Eng. J. 2016, 283, 816.
[51] Jia, L.; Wang, D. H.; Huang, Y. X.; Xu, A. W.; Yu, H. Q. J. Phys. Chem. C 2011, 115, 11466.
[52] Guo, R. Q.; Fang, L.; Dong, W.; Zheng, F. G.; Shen, M. R. J. Mater. Chem. 2011, 21, 18645.
[53] Mondal, S.; Sunhu, S.; Bhattacharya, S.; Saha, S. K. J. Phys. Chem. C 2015, 119, 27749.
/
| 〈 |
|
〉 |