

Preparation of Ni/C Core-shell Nanoparticles through MOF Pyrolysis for Phenylacetylene Hydrogenation Reaction
Received date: 2017-07-26
Online published: 2017-10-24
Supported by
Project supported by the National Natural Science Foundation of China (Nos. 21373038, 21403026), the Natural Science Foundation of Liaoning Province in China (No. 2015021014), and the Fundamental Research Funds for the Central Universities (No. DUT16RC(4)03).
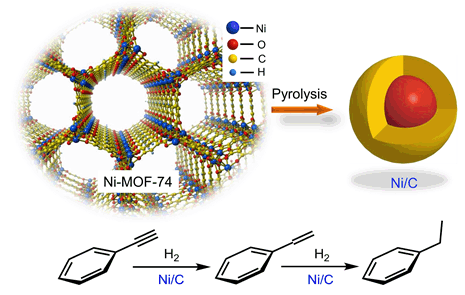
A series of Ni/C core-shell nano catalysts with abundant mesoporous and uniform size were prepared by Ni-MOF-74 pyrolysis. The Ni-MOF-74 was synthesized via hydrothermal method with nickel acetate and 2,5-dihydroxyterephthalic acid (DHTA) as raw materials. The pyrolysis process was carried out in a tube furnace under Argon (Ar) atmosphere with a heating rate of 2℃/min. Completed pyrolytic product Ni/C can be obtained by extending the pyrolysis time (6 h) at 400℃ or increasing the pyrolysis temperature (≥ 500℃) based on the TG result. Moreover, the particle size of Ni/C varied with pyrolysis temperature from 3 nm (500℃) to 17 nm (800℃). The TEM images and Ar ion sputtering XPS indicated a core-shell structure of the pyrolysis product. Nickel species can be stable in the form of nickel (Ni0) due to the electronic properties regulating and confinement effect of the carbon shell. Moreover, the carbon shell greatly weaken the interaction between particles, which is favorable for the dispersion of the catalyst in the reaction system. H2-TPR results show that the interaction between nickel and amorphous carbon increases with the pyrolysis temperature, which is unfavorable to the interaction between Ni species and the reactant. The phenylacetylene (PA) hydrogenation reaction was carried out with 0.2 g catalyst, 10 mL of 1 mol/L ethanolic phenylacetylene solution and 1.0 MPa H2 in a 50 mL high-pressure autoclave under 50℃. Ni/C exhibits excellent catalytic activity and recyclability in phenylacetylene (PA) hydrogenation. In addition, we compared the activity of Ni/C with several reported catalyst system and found their activity increases in the order of Ni, NiSix, supported Ni2Si, Ni/C, Pd and Pt. With an activity of up to 0.833 mmol•min-1•gcat.-1 at 50℃ (Ni/C-400-6, Ni/C-500-2), Ni/C is the most promising transition metal catalyst that can be comparable with noble metal.
Guo Xiaoling , Chen Xiao , Su Dangsheng , Liang Changhai . Preparation of Ni/C Core-shell Nanoparticles through MOF Pyrolysis for Phenylacetylene Hydrogenation Reaction[J]. Acta Chimica Sinica, 2018 , 76(1) : 22 -29 . DOI: 10.6023/A17070339
[1] (a) Wu, Z. L.; Cravotto, G.; Gaudino, E. C.; Giacomino, A.; Medlock, J.; Bonrath, W. Ultrason. Sonochem. 2017, 35, 664.
(b) Yang, K. X.; Chen, X.; Guan, J. C.; Liang, C. H. Catal. Today 2015, 246, 176.
[2] Wang, B.; Sun, L. M.; Ma, H. W.; Zheng, Y. D.; Hu, X. L.; Yang, H. Q.; Liang, S. Q. Contemp. Chem. Ind. 2015, 2048. (王斌, 孙利民, 马好文, 郑云弟, 胡晓丽, 杨红强, 梁顺琴, 当代化工, 2015, 2048.)
[3] Berenblyum, A. S.; Al-Wadhaf, H. A.; Katsman, E. A. Petrol. Chem. 2015, 55, 118.
[4]
(a) Mastalir, Á.; Király, Z. J. Catal. 2003, 220, 372;
(b) Erokhin, A. V.; Lokteva, E. S.; Yermakov, A. Y.; Boukhvalov, D. W.; Maslakov, K. I.; Golubina, E. V.; Uimin, M. A. Carbon 2014, 74, 291.
[5] Su, D. S.; Perathoner, S.; Centi, G. Chem. Rev. 2013, 113, 5782.
[6] Li, S. Q.; Liu, J. T.; Sun, F. X.; Wang, W. M.; Cheng, Y. L. CN101475438, 2009[Chem. Abstr. 2009, 35, 840049].
[7] Beyhaghi, M.; Kiani-Rashid, A. R.; Kashefi, M.; Khaki, J. V.; Jonsson, S. Appl. Surf. Sci. 2015, 344, 1.
[8]
(a) Cho, G. S.; Lim, J. K.; Choe, K. H.; Lee, W. Mater. Sci. Forum 2010, 658, 360;
(b) Wu, C. Z.; Yao, X. D.; Zhang, H. Int. J. Hydrogen Energy 2010, 35, 247.
[9] Manukyan, A.; Mirzakhanyan, A.; Sajti, L.; Khachaturyan, R.; Kaniukov, E.; Lobanovsky, L.; Sharoyan, E. Nano 2015, 10, 7.
[10] Meng, Z. Q.; Li, X. B.; Xiong, Y. J.; Zhan, J. T. Nonferr. Metal. Soc. 2012, 22, 2719.
[11] Li, X.; Cheng, H.; Fan, J. Powder Metall. Technol. 2009, 27, 142.
[12] Shen, K.; Chen, X.; Chen, J.; Li, Y. ACS Catal. 2016, 6, 5887.
[13] Meek, S. T.; Greathouse, J. A.; Allendorf, M. D. Adv. Mater. 2011, 23, 249.
[14] Dietzel, P. D.; Panella, B.; Hirscher, M.; Blom, R.; Fjellvag, H. Chem. Commun. 2006, 959.
[15] Dietzel, P. D.; Morita, Y.; Blom, R.; Fjellvag, H. Angew. Chem., Int. Ed. 2005, 44, 6354.
[16] Grant Glover, T.; Peterson, G. W.; Schindler, B. J.; Britt, D.; Yaghi, O. Chem. Eng. Sci. 2011, 66, 163.
[17] An, C.; Liu, G.; Li, L.; Wang, Y.; Chen, C.; Wang, Y.; Jiao, L.; Yuan, H. Nanoscale 2014, 6, 3223.
[18] Zhou, L.; Zhang, T.; Tao, Z.; Chen, J. Nano Res. 2014, 7, 774.
[19]
(a) Paul, R.; Sharma, M. K.; Chatterjee, R.; Hussain, S.; Bhar, R.; Pal, A. K. Appl. Surf. Sci. 2012, 258, 5850;
(b) Wang, H.; Cao, Y.; Zou, G.; Yi, Q.; Guo, J.; Gao, L. ACS Appl. Mater. Interfaces 2017, 9, 60;
(c) Kovacs, G. J.; Bertoti, I.; Radnoczi, G. Thin Solid Films 2008, 516, 7942.
[20]
(a) Leng, Y. G.; Shao, H. Y.; Wang, Y. T.; Suzuki, M.; Li, X. G. J. Nanosci. Nanotechnol. 2006, 6, 221;
(b) Hasegawa, M.; Sugawara, K.; Suto, R.; Sambonsuge, S.; Teraoka, Y.; Yoshigoe, A.; Filimonov, S.; Fukidome, H.; Suemitsu, M. Nanoscale Res. Lett. 2015, 10, 421.
[21] Golubina, E. V.; Lokteva, E. S.; Erokhin, A. V.; Veligzhanin, A. A.; Zubavichus, Y. V.; Likholobov, V. A.; Lunin, V. V. J. Catal. 2016, 344, 90.
[22] Guo, J. X.; Liang, J.; Chu, Y. H.; Sun, M. C.; Yin, H. Q.; Li, J. J. Appl. Catal. A: Gen. 2012, 421, 142.
[23] Park, S. J.; Jung, W. Y. J. Colloid Interface Sci. 2002, 250, 93.
[24] Wang, Z. M.; Yamashita, N.; Wang, Z. X.; Hoshinoo, K.; Kanoh, H. J. Colloid Interface Sci. 2004, 276, 143.
[25] Ortega, K. F.; Arrigo, R.; Frank, B.; Schlogl, R.; Trunschke, A. Chem. Mater. 2016, 28, 6826.
[26] Oswald, S.; Bruckner, W. Surf. Interface Anal. 2004, 36, 17.
[27] Carraro, P. M.; Blanco, A. A. G.; Soria, F. A.; Lener, G.; Sapag, K.; Eimer, G. A.; Oliva, M. I. Microporous Mesoporous Mater. 2016, 231, 31.
[28] Cheng, C. B.; Shen, D. K.; Xiao, R.; Wu, C. F. Fuel 2017, 189, 419.
[29] Rodriguez-Gomez, A.; Caballero, A. ChemNanoMat 2017, 3, 94.
[30] Liu, L. J.; Lou, H.; Chen, M. Int. J. Hydrogen Energy 2016, 41, 14721.
[31] Hu, D.; Gao, J.; Ping, Y.; Jia, L.; Gunawan, P.; Zhong, Z.; Xu, G.; Gu, F.; Su, F. Ind. Eng. Chem. Res. 2012, 51, 4875.
[32] Yang, K. X.; Chen, X.; Wang, L.; Zhang, L. L.; Jin, S. H.; Liang, C. H. ChemCatChem 2017, 9, 1337.
[33]
(a) Carturan, G.; Facchin, G.; Gottardi, V.; Guglielmi, M.; Navazio, G. J. Non-Cryst. Solids 1982, 48, 219;
(b) Costa, M.; Pelagatti, P.; Pelizzi, C.; Rogolino, D. J. Mol. Catal. A: Chem. 2002, 178, 21.
[34] Chen, X.; Li, M.; Guan, J.; Wang, X.; Williams, C. T.; Liang, C. Ind. Eng. Chem. Res 2012, 51, 3604.
[35] Kulikov, L. A.; Terenina, M. V.; Kryazheva, I. Y.; Karakhanov, E. A. Petro. Chem. 2017, 57, 222.
[36] Zhang, W.; Wang, F. S.; Li, X. L.; Liu, Y. S.; Liu, Y.; Ma, J. T. Appl. Surf. Sci. 2017, 404, 398.
[37] Boronoev, M. P.; Subbotina, E. S.; Kurmaeva, A. A.; Kardasheva, Y. S.; Maksimov, A. L.; Karakhanov, E. A. Petrol. Chem. 2016, 56, 109.
[38]
(a) Chen, W.; Pan, X. L.; Bao, X. H. J. Am. Chem. Soc. 2007, 129, 7421;
(b) Chen, W.; Pan, X. L.; Willinger, M. G.; Su, D. S.; Bao, X. H. J. Am. Chem. Soc. 2006, 128, 3136.
[39] Fu, Q.; Bao, X. H. Chin. J. Catal. 2015, 36, 517.
[40] Deng, J.; Ren, P. J.; Deng, D. H.; Bao, X. H. Angew. Chem. Int. Ed. 2015, 54, 2100.
[41] Chen, X.; Li, M.; Guan, J.; Wang, X.; Williams, C. T.; Liang, C. Ind. Eng. Chem. Res. 2012, 51, 3604.
/
| 〈 |
|
〉 |