

Synthesis and Study of Hypoxia-Responsive Micelles Based on Hyaluronic Acid
Received date: 2017-07-25
Online published: 2017-11-10
Supported by
Project supported by the China National Funds for Distinguished Young Scientists (No. 51325302), the National Natural Science Foundation of China (No. 51533007), the Major State Basic Research Development Program of China (No. 2013CB933002), the Open Project Program of the State Key Lab of Molecular Engineering of Polymers, the Fudan University (No. K2017-10) and the Natural Science Foundation of Hubei Province (No. 2015CFB304).
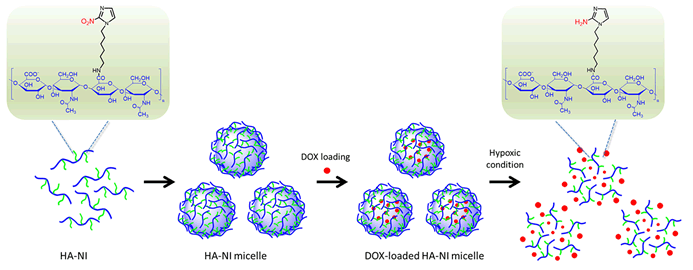
Hypoxia is a hallmark of tumor. Based on this feature, a hypoxia-responsive drug delivery system combined with tumor-targeting was developed. HA-NI conjugates were prepared by grafting the carboxyl group of hyaluronic acid (HA) with an amine group of nitroimidazole (NI) derivative in the presence of 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC) and N-hydroxysuccinimide (NHS). The structure of HA-NI conjugates was confirmed by FT-IR and 1H NMR, the degree of substitution (DS) of NI derivative was also calculated based on 1H NMR. Amphiphilic HA-NI conjugates could self-assemble into micelles by ultrasonic method. The size and morphology of micelles were characterized by dynamic light scattering (DLS), atomic force microscope (AFM) and transmission electron microscopy (TEM), the stability of micelles was also investigated by DLS. It was found the size of micelles was in the range of 80~220 nm while the DS decreased from 6.5% to 3.6%. Doxorubicin (DOX) was encapsulated in micelles, and DOX-loaded micelles had smaller sizes compared with blank micelles. Drug-loading (DL) and entrapment efficiency (EE) were obtained by UV-Vis analysis and increased with the increasing DS. Under hypoxic condition, micelles became bigger and size distribution of micelles became wider, it was clear to observe the destruction of micelles by AFM and TEM. UV spectrum revealed the characteristic peak belonging to NI at 325 nm disappeared and Zeta potential increased from -30.6±0.4 mV to -24.9±0.5 mV 6 h later. In vitro drug release studies demonstrated that DOX was released from HA-NI micelles in a hypoxia-dependent manner:micelles were sufficiently stable at normoxic condition while accomplished a rapid drug release under hypoxic condition.
Key words: drug delivery system; hypoxia-responsive; hyaluronic acid; nitroimidazole; micelles
Zhang Bei , Chang Baisong , Sun Taolei . Synthesis and Study of Hypoxia-Responsive Micelles Based on Hyaluronic Acid[J]. Acta Chimica Sinica, 2018 , 76(1) : 35 -42 . DOI: 10.6023/A17070336
[1] Im, J. H.; Seong, J.; Lee, I. J.; Park, J. S.; Yoon, D. S.; Kim, K. S.; Lee, W. J.; Park, K. R. Cancer. Res. Treat. 2016, 48, 583.
[2] Zhang, W. G.; Mao, J. H.; Zhu, W.; Jain, A. K.; Liu, K.; Brown, J. B.; Karpenb, G. H. Nat. Commun. 2016, 7, 12619.
[3] Zhang, P.; Qian, X. P.; Zhang, Z. K.; Li, C.; Xie, C.; Wu, W.; Jiang, X. Q. ACS. Appl. Mater. Interfaces 2017, 9, 5768.
[4] Mura, S.; Nicolas, J.; Couvreur, P. Nat. Mater. 2013, 12, 991.
[5] Xu, T. L.; Gao, W.; Xu, L. P.; Zhang, X. J.; Wang, S. T. Adv. Mater. 2016, 29, 1603250.
[6] Liu, C. Y.; Hu, J. H.; Yang, D.; Yang, W. L. Acta Chim. Sinica 2009, 67, 843. (刘聪颖, 胡建华, 杨东, 杨武利, 化学学报, 2009, 67, 843.)
[7] Zhao, W. J.; Qiao, Z. Y.; Duan, Z. Y.; Wang, H. Acta Chim. Sinica 2016, 74, 234. (赵文静, 乔增莹, 段中余, 王浩, 化学学报, 2016, 74, 234.)
[8] Hu, Q.; Li, Y. X.; Wang, J. Y.; Li, Y. P. Acta Chim. Sinica 2015, 73, 416. (胡齐, 李玉祥, 王静媛, 李亚鹏, 化学学报, 2015, 73, 416.)
[9] Wilson, W. R.; Hay, M. P. Nat. Rev. Cancer. 2011, 11, 393.
[10] Zheng, X. C.; Wang, X.; Mao, H.; Wu, W.; Liu, B. R.; Jiang, X. Q. Nat. Commun. 2015, 6, 5834.
[11] Höckel, M.; Vaupel, P. J. Natl. Cancer. Inst. 2001, 93, 266.
[12] Bertout, J. A.; Patel, S. A.; Simon, M. C. Nat. Rev. Cancer. 2008, 8, 967.
[13] Harris, A. L. Nat. Rev. Cancer. 2002, 2, 38.
[14] Thambi, T.; Park, J. H.; Lee, D. S. Chem. Commun. 2016, 52, 8492.
[15] Thambi, T.; Son, S.; Lee, D. S.; Park, J. H. Acta Biomater. 2016, 29, 261.
[16] Xu, K. H.; Wang, F.; Pan, X. H.; Liu, R. P.; Ma, J.; Kong, F. P.; Tang, B. Chem. Commun. 2013, 49, 2554.
[17] Herrlich, P.; Sleeman, J.; Wainwright, D.; König, H.; Sherman, L.; Hilberg, F.; Ponta, H. Cell. Adhes. Commun. 1998, 6, 141.
[18] Hall, C. L.; Yang, B.; Yang, X.; Zhang, S.; Turley, M.; Samuel, S.; Lange, L. A.; Wang, C.; Curpen, G. D.; Savani, R. C.; Greenberg, A. H.; Turley, E. A. Cell 1995, 82, 19.
[19] Choi, K. Y.; Saravanakumar, G.; Park, J. H.; Park, K. Colloids Surf., B:Biointerfaces 2012, 99, 82.
[20] Liu, Y. H.; Sun, J.; Cao, W.; Yang, J. H.; Lian, H.; Li, X.; Sun, Y. H.; Wang, Y. J.; Wang, S. L.; He, Z. G. Int. J. Pharm. 2011, 421, 160.
[21] Oh, E. J.; Park, K.; Kim, K. S.; Kim, J.; Yang, J. A.; Kong, J. H.; Lee, M. Y.; Hoffman, A. S.; Hahn, S. K. J. Control. Release 2010, 141, 2.
[22] Liu, Y. H.; Zhou, C. M.; Wang, W. P.; Yang, J. H.; Wang, H.; Hong, W.; Huang, Y. Mol. Pharm. 2016, 13, 4209.
[23] Chen, Yi.; Li, H. H.; Deng, Y. Y.; Sun, H. F.; Ke, X.; Ci, T. Y. Acta Biomater. 2017, 51, 374.
[24] Šmejkalová, D.; Muthný, T.; Nešporová, K.; Hermannová, M.; Achbergerová, E.; Huerta-Angeles, G.; Svoboda, M.; Cepa, M.; Machalová, V.; Luptáková, D.; Velebný, V. Carbohydr. Polym. 2017, 156, 86.
[25] Luo, Y.; Prestwich, G. D. Bioconjugate Chem. 1999, 10, 755.
[26] Bailly, N.; Thomas, M.; Klumperman, B. Biomacromolecules 2012, 13, 4109.
[27] Kratohvil, J. P.; Hsu, W. P.; Kwok, D. I. Langmuir 2002, 2, 256.
[28] Song, S. S.; Chen, F.; Qi, H.; Li, F.; Xin, T. G.; Xu, J. W.; Ye, T. T.; Sheng, N. C.; Yang, X. G.; Pan, W. S. Pharm. Res. 2014, 31, 1032.
[29] Chen, Z. J.; He, N.; Chen, M. H.; Zhao, L.; Li, X. H. Acta Biomater. 2016, 43, 195.
[30] Lin, Q. N.; Bao, C. Y.; Yang, Y. L.; Liang, Q. N.; Zhang, D. S.; Cheng, S. Y.; Zhu, L. Y. Adv. Mater. 2013, 25, 1981.
[31] Hu, L. Q. M.S. Dissertation, Hunan University, Changsha, 2012 (胡利强, 硕士论文, 湖南大学, 长沙, 2012.)
/
| 〈 |
|
〉 |