

Oxidative Iodohydroxylation of Olefins with DMSO
Received date: 2017-10-03
Online published: 2017-11-22
Supported by
Project supported by the National Natural Science Foundation of China (Nos. 21325206, 21632001 and 21602005) and the State Key Laboratory of Drug Research.
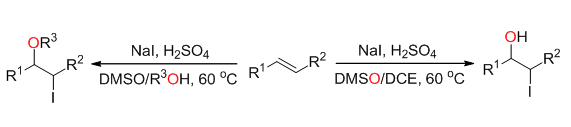
Halohydrins bearing a hydroxyl and halide functional group, are privileged building blocks in organic synthesis and could be conveniently converted to other significant organic intermediates such as azidoalcohols, aminoalcohols, and epoxides, all of which are widely used in the synthesis of highly value-added chemicals. Among the approaches to halohydrins, the halohydroxylation of olefins provides a direct and efficient approach. The synthesis of bromohydrins has achieved great progress in recent years. However, the approaches to iodohydrins are still very limited. Our previous studies revealed that DMSO could oxidize halo anions to halo cations under acidic conditions. As our continuous development DMSO-based reactions, we report the iodohydroxylation of olefins by using DMSO and HI generated in situ. In this transformation, DMSO performed versatile roles as an oxidant, a solvent and an oxygen source. This reaction featured with simple operation, mild reaction condition, and wild substrate scope, and provided an efficient method to synthesize iodohydrins. Furthermore, the iodoetheration of olefins was also realized by using DMSO and alcohol as the solvent. A representative procedure for this reaction is as following:The mixture of alkene (0.5 mmol), NaI (0.6 mmol), conc. H2SO4 (1.0 mmol), DMSO (1 mL) and DCE (1 mL) were stirred at 60℃ under air. TCL monitor the reaction, and the product had a clear spot in phosphomolybdic acid chromogenic agent. After the reaction was completed, saturated solution of Na2S2O3 (0.5 mL) was added into the system to consume the extra I2. After cooling down to room temperature, the mixture was diluted with water (10 mL) and extracted with ethyl acetate (10 mL×3). The combined organic extract was washed with saturated solution of NaCl (15 mL), dried over MgSO4, and evaporated in vacuo. The residue was purified by chromatography on silica gel (petroleum ether/ethyl acetate) to afford the desired product.
Key words: DMSO; olefin; oxidation; iodohydroxylation; iodohydrin
Li Xinwei , Song Song , Jiao Ning . Oxidative Iodohydroxylation of Olefins with DMSO[J]. Acta Chimica Sinica, 2017 , 75(12) : 1202 -1206 . DOI: 10.6023/A17100448
[1] Gribble, G. W. J. Chem. Educ. 2004, 81, 1441.
[2] Dagani, M. J.; Barda, H. J.; Benya, T. J.; Sanders, D. C. Bromine Compounds in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2002.
[3] Neilson, A. H. Organic Bromine and Iodine Compounds in The Handbook of Environmental Chemistry, Springer, Berlin, Heidelberg, 2003.
[4]
(a) Smith, J. G.; Fieser, M. In Fieser and Fieser's Reagent for Organic Synthesis, Vol. 1~12, John Wiley and Sons, New York, 1990.
(b) Grogan, G. Annu. Rep. Prog. Chem., Sect. B 2009, 105, 206.
(c) Mahdi, T.; Stephan, D. W. J. Am. Chem. Soc. 2014, 136, 15809.
[5]
(a) Sels, B. F.; De Vos, D. E.; Jacobs, P. A. J. Am. Chem. Soc. 2001, 123, 8350.
(b) Pandit, P.; Gayen, K. S.; Khamarui, S.; Chatterjee, N.; Maiti, D. K. Chem. Commun. 2011, 47, 6933.
(c) Dewkar, G. K.; Narina, S. V.; Sudalai, A. Org. Lett. 2003, 5, 4501.
(d) Yang, X.; Wu, J.; Mao, X.; Jamison, T. F.; Hatton, T. A. Chem. Commun. 2014, 50, 3245.
(e) Darensbourg, D. J.; Wilson, S. J. J. Am. Chem. Soc. 2011, 133, 18610.
(f) Kozhushkov, S. I.; Yufit, D. S.; de Meijere, A. Adv. Synth. Catal. 2005, 347, 255.
(g) Sels, B.; De Vos, D.; Buntinx, M.; Pierard, F.; Kirsch-De Mesmaeker, A.; Jacobs, P. Nature 1999, 400, 855.
(h) Ashikari, Y.; Shimizu, A.; Nokami, T.; Yoshida, J. J. Am. Chem. Soc. 2013, 135, 16070.
[6]
(a) Hajra, S.; Karmakar, A.; Bhowmick, M. Tetrahedron 2005, 61, 2279.
(b) Schmid, G. H.; Gordon, J. W. J. Org. Chem. 1983, 48, 4010.
(c) Mahajan, V. A.; Shinde, P. D.; Gajare, A. S.; Karthikeyan, M.; Wakharkar, R. D. Green Chem. 2002, 4, 325.
(d) Barluenga, J.; Rodriguez, M. A.; Campos, P. J.; Asensio, G. J. Chem. Soc., Chem. Commun. 1987, 1491.
[7]
(a) Barluenga, J.; Gonzalez, J. M.; Campos, P. J.; Asensio, G. Angew. Chem. 1985, 97, 341.
(b) Rao, D. S.; Reddy, T. R.; Babachary, K.; Kashyap, S. Org. Biomol. Chem. 2016, 14, 7529.
[8] For selected reviews on enzyme-catalyzed oxidative halogenations, see:
(a) Vaillancourt, F. H.; Yeh, E.; Vosburg, D. A.; Garneau-Tsodikova, S.; Walsh, C. T. Chem. Rev. 2006, 106, 3364.
(b) Wischang, D.; Brücher O.; Hartung, J. Coord. Chem. Rev. 2011, 255, 2204.
(c) Lewis, J. C.; Coelho, P. S.; Arnold, F. H. Chem. Soc. Rev. 2011, 40, 2003.
[9]
(a) Agrawal, M. K.; Adimurthy, S.; Ganguly, B.; Ghosh, P. K. Tetrahedron 2009, 65, 2791.
(b) Chakraborty, N.; Santra, S.; Kundu, S. K.; Hajra, A.; Zyryanov, G. V.; Majee, A. RSC Adv. 2015, 5, 56780.
[10]
(a) Barluenga, J.; Marco-Arias, M.; Gonzalez-Bobes, F.; Ballesteros, A.; Gonzalez, J. M. Chem.-Eur. J. 2004, 10, 1677.
(b) Stavber, G.; Iskra, J.; Zupan, M.; Stavber, S. Adv. Synth. Catal. 2008, 350, 2921.
[11]
(a) Martin, D.; Weise, A.; Niclas, H. J. Angew. Chem., Int. Ed. 1967, 6, 318.
(b) Sun, P.; Yang, D.; Wei, W.; Jiang, L.; Wang, Y.; Dai, T.; Wang, H. Org. Chem. Front. 2017, 4, 1367.
(c) Yang, X.-Q.; Huang, K.-M.; Jia, G.-Z. Acta Chim. Sinica 2008, 66, 1107(in Chinese). (杨晓庆, 黄卡玛, 贾国柱, 化学学报, 2008, 66, 1107.)
(d) Xie, K.; Qiao, S.; Cheng, C. Acta Chim. Sinica 2009, 67, 231(in Chinese). (谢昆, 乔澍, 程聪, 化学学报, 2009, 67, 231.)
(e) Liu, J.; Wang, W.; Wang, R.; Gu, L. Chin. J. Chem. 2015, 33, 559.
(f) Yang, J.; Yin, W.; Liu, R.; Chu, C. Chin. J. Chem. 2012, 30, 2786.
[12]
(a) Omura, K.; Sharma, A. K.; Swern, D. J. Org. Chem. 1976, 41, 957.
(b) Pfitzner, K. E.; Moffatt, J. G. J. Am. Chem. Soc. 1963, 85, 3027.
(c) Corey, E. J.; Chaykovsky, M. J. Am. Chem. Soc. 1962, 84, 867.
[13]
(a) Song, S.; Huang, X.; Liang, Y.-F.; Yuan, Y.; Li, X.; Jiao, N. Green Chem. 2015, 17, 2727.
(b) Tomita, R.; Yasu, Y.; Koike, T.; Akita, M. Angew. Chem., Int. Ed. 2014, 53, 7144.
(c) Mupparapu, N.; Khan, S.; Battula, S.; Kushwaha, M.; Gupta, A. P.; Ahmed, Q. N.; Vishwakarma, R. A. Org. Lett. 2014, 16, 1152.
(d) Wu, X.; Gao, Q.; Liu, S.; Wu, A. Org. Lett. 2014, 16, 2888.
(e) Gao, Q.; Wu, X.; Liu, S.; Wu, A. Org. Lett. 2014, 16, 1732.
(f) Ashikari, Y.; Nokami, T.; Yoshida, J. J. Am. Chem. Soc. 2011, 133, 11840.
(g) Ashikari, Y.; Nokami, T.; Yoshida, J. Org. Lett. 2012, 14, 938.
(h) Xu, R.; Wan, J.-P.; Mao, H.; Pan, Y. J. Am. Chem. Soc. 2010, 132, 15531.
(i) Liang, Y.-F.; Wu, K.; Song, S.; Li, X.; Huang, X.; Jiao, N. Org. Lett. 2015, 17, 876.
(j) Liang, Y.-F.; Li, X.; Wang, X.; Zou, M.; Tang, C.; Liang, Y.; Song, S.; Jiao, N. J. Am. Chem. Soc. 2016, 138, 12271.
[14]
(a) Jiang, X.; Wang, C.; Wei, Y.; Xue, D.; Liu, Z.; Xiao, J. Chem.-Eur. J. 2014, 20, 58.
(b) Qian, J.; Zhang, Z.; Liu, Q.; Liu, T.; Zhang, G. Adv. Synth. Catal. 2014, 356, 3119.
(c) Ren, X.; Chen, J.; Chen, F.; Cheng, J. Chem. Commun. 2011, 47, 6725.
(d) Lv, Y.; Li, Y.; Xiong, T.; Pu, W.; Zhang, H.; Sun, K.; Liu, Q.; Zhang, Q. Chem. Commun. 2013, 49, 6439.
(e) Wu, X.-F.; Natte, K. Adv. Synth. Catal. 2016, 358, 336.
(f) Jones-Mensah, E.; Karki, M.; Magolan, J. Synthesis 2016, 48, 1421.
(g) Shen, T.; Huang, X.; Liang, Y.-F.; Jiao, N. Org Lett. 2015, 17, 6186.
[15]
(a) Liu, F.-L.; Chen, J.-R.; Zou, Y.-Q.; Wei, Q.; Xiao, W.-J. Org. Lett. 2014, 16, 3768.
(b) Mal, K.; Sharma, A.; Maulik, P. R.; Das, I. Chem.-Eur. J. 2014, 20, 662.
(c) Gao, Q.; Wu, X.; Li, Y.; Liu, S.; Meng, X.; Wu, A. Adv. Synth. Catal. 2014, 356, 2924.
(d) Chu, L.; Yue, X.; Qing, F.-L. Org. Lett. 2010, 12, 1644.
(e) Yin, G.; Zhou, B.; Meng, X.; Wu, A.; Pan, Y. Org. Lett. 2006, 8, 2245.
(f) Hu, G.; Xu, J.; Li, P. Org. Lett. 2014, 16, 6036.
(g) Gao, X.; Pan, X.; Gao, J.; Huang, H.; Yuan, G.; Li, Y. Chem. Commun. 2015, 51, 210.
(h) Jiang, Y.; Loh, T.- P. Chem. Sci. 2014, 5, 4939.
(i) Gao, X.; Pan, X.; Gao, J.; Jiang, H.; Yuan, G.; Li, Y. Org. Lett. 2015, 17, 1038.
[16]
(a) Song, S.; Li, X.; Sun, X.; Yuan, Y.; Jiao, N. Green Chem. 2015, 17, 3285.
(b) Song, S.; Sun, X.; Li, X.; Yuan, Y.; Jiao, N. Org. Lett. 2015, 17, 2886.
[17]
(a) Gao, Q.; Liu, S.; Wu, X.; Wu, A. Org. Lett. 2014, 16, 4582.
(b) Liu, S.; Xi, H.-L.; Zhang, J.-J.; Wu, X.; Gao, Q.-H.; Wu, A.-X. Org. Biomol. Chem. 2015, 13, 8807.
(c) Gao, Q.; Zhang, J.; Wu, X.; Liu, S.; Wu, A. Org. Lett. 2015, 17, 134.
(d) Gao, Q.; Liu, S.; Wu, X.; Zhang, J.; Wu, A. Org. Lett. 2015, 17, 2960.
(e) Zhang, J.; Gao, Q.; Wu, X.; Geng, X.; Wu, Y.-D.; Wu, A. Org. Lett. 2016, 18, 1686.
(f) Fan, W.; Yang, Z.; Jiang, B.; Li, G. Org. Chem. Front. 2017, 4, 1091.
(g) Zhang, Z.; Xie, C.; Tan, X.; Song, G.; Wen, L.; Gao, H.; Ma, C. Org. Chem. Front. 2015, 2, 942.
(h) Wu, W.; An, Y.; Li, J.; Yang, S.; Zhu, Z.; Jiang, H. Org. Chem. Front. 2017, 4, 1751.
(i) Wu, Y.-D.; Geng, X.; Gao, Q.; Zhang, J.; Wu, X.; Wu, A.-X. Org. Chem. Front. 2016, 3, 1430.
/
| 〈 |
|
〉 |