

Controllable Synthesis of One-dimensional Cryptomelane-type Manganese Dioxide and Its Electrochemical Performance
Received date: 2017-09-13
Online published: 2018-01-09
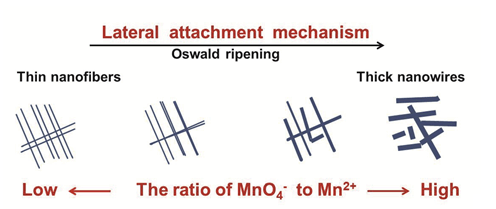
Cryptomelane-type manganese dioxide (OMS-2) is a very important nanomaterial in electrochemistry. Its intrinsic properties can be tailored by controlling shape or size. The diameter of one-dimensional OMS-2 nanomaterial is an important parameter in controllable synthesis and electrochemistry applications. Generally, the control of the diameter of one-dimensional OMS-2 nanomaterial can be realized by cosolvents or surfactants, even other special methods. In this paper, without any acid added, a series of one-dimensional OMS-2 nanomaterial with different diameters were synthesized by adjusting the ratio of potassium permanganate to manganese sulfate monohydrate in the aqueous solution with the conditional reflux method. The typical samples were characterized in detail by N2 adsorption-desorption analyses (BET), Fourier transform infrared spectroscopy (FT-IR), X-ray diffraction (XRD), transmission electron microscope (TEM) and hydrogen temperature-programmed reduction (H2-TPR). The results reconfirmed that the growth of OMS-2 nanofibers and nanowires mainly followed the lateral attachment mechanism. The role of Oswald ripening in the growth of one-dimensional OMS-2 nanomaterials was making two or more primary thinner nanofibers or nanowires welded together. In the synthesis process, all the conditions were strictly controlled. The samples synthesized at low ratio of MnO4- to Mn2+ showed thinner and longer nanofibers or nanowires, and the samples synthesized at high ratio of MnO4- to Mn2+ exhibited higher diameter. Therefore, it can be concluded that MnO4- can promote the lateral growth of one-dimensional OMS-2 nanomaterials and Mn2+ tends to promote the longitudinal growth. In the electrochemical tests, when the ratio of potassium permanganate to manganese sulfate monohydrate increased from 0.15 to 1.80, the specific capacitance of one-dimensional OSM-2 nanomaterials decreased gradually. Therefore, the specific capacitance of one-dimensional OSM-2 nanomaterial was directly related to their diameters. The smaller the diameter is, the larger the capacitance is. The specific capacitance of MnO-15, MnO-45, MnO-112 and MnO-180 was 375, 230, 144 and 77 F/g, respectively. The result of galvanostatic charge and discharge of four samples at the current density of 1 A/g in 1 mol/L Na2SO4 solution was consistent with the cyclic voltammetry.
Key words: manganese oxide; one-dimensional; diameter; electrochemistry
Zhang Chengming , Pang Xin , Wang Yongzhao . Controllable Synthesis of One-dimensional Cryptomelane-type Manganese Dioxide and Its Electrochemical Performance[J]. Acta Chimica Sinica, 2018 , 76(2) : 133 -137 . DOI: 10.6023/A17090418
[1] Thenuwara, A. C.; Shumlas, S. L.; Attanayake, N. H.; Cerkez, E. B.; McKendry, I. G.; Frazer, L.; Borguet, E.; Kang, Q.; Zdilla, M. J.; Sun, J. Langmuir 2015, 31, 12807.
[2] Liu, J.; Younesi, R.; Gustafsson, T.; Edström, K.; Zhu, J. Nano Energy 2014, 10, 19.
[3] Ran, F.; Fan, H.; Wang, L.; Zhao, L.; Tan, Y.; Zhang, X.; Kong, L.; Kang, L. J. Energ. Chem. 2013, 22, 928.
[4] Zhang, K.; Han, P.; Gu, L.; Zhang, L.; Liu, Z.; Kong, Q.; Zhang, C.; Dong, S.; Zhang, Z.; Yao, J.; Xu, H.; Cui, G.; Chen, L. ACS Appl. Mater. Interfaces 2012, 4, 658.
[5] Hu, B.; Chen, C. H.; Frueh, S. J.; Jin, L.; Joesten, R.; Suib, S. L. J. Phys. Chem. C 2010, 114, 9835.
[6] Yang, C.; Gong, Z.; Zhao, W.; Yang, Y. Acta Chim. Sinica 2017, 75, 212(in Chinese). (杨春, 龚正良, 赵文高, 杨勇, 化学学报, 2017, 75, 212.)
[7] Liu, L.; Qi, X.; Hu, Y.; Chen, L.; Huang, X. Acta Chim. Sinica 2017, 75, 218(in Chinese). (刘丽露, 戚兴国, 胡勇胜, 陈立泉, 黄学杰, 化学学报, 2017, 75, 218.)
[8] Brock, S. L.; Duan, T. Z. R. Chem. Mater. 1998, 10, 2619.
[9] Su, Z.;Ye, S.; Wang, Y. Acta Chim. Sinica 2009, 67, 2413(in Chinese). (粟智, 叶世海, 王永龙, 化学学报, 2009, 67, 2413.)
[10] Debart, A.; Paterson, A. J.; Bao, J.; Bruce, P. G. Angew. Chem. Int. Ed. 2008, 47, 4521.
[11] Nyutu, E. K.; Chen, C. H.; Sithambaram, S.; Crisostomo,V. M. B.; Suib, S. L. J. Phys. Chem. C 2008, 112, 6786.
[12] Kumar, N.; Dineshkumar, P.; Rameshbabu, R.; Sen, A. Mater. Lett. 2015, 158, 309.
[13] Sampanthar, J. T.; Dou, J.; Joo, G. G.; Widjaja, E.; Eunice, L. Q. H. Nanotechnology 2007, 18, 25601.
[14] Xu, N.; Ma, X.; Qiao, S.; Yuan, J.; Liu, Z. Acta Chim. Sinica 2009, 67, 2566(in Chinese). (许乃才, 马向荣, 乔山峰, 袁佳琦, 刘宗怀, 化学学报, 2009, 67, 2566.)
[15] Hernández, W. Y.; Centeno, M. A.; Romero Sarria, F.; Ivanova, S.; Montes, M.; Odriozola, J. A. Catal. Today 2010, 157, 160.
[16] Li, Y.; Wang, J.; Zhang, Y.; Banis, M. N.; Liu, J.; Geng, D.; Li, R.; Sun, X. J. Colloid. Interf. Sci. 2012, 369, 123.
[17] Sun, H.; Liu, Z.; Chen, S.; Quan, X. Chem. Eng. J. 2015, 270, 58.
[18] Gao, T.; Glerup, M.; Krumeich, F.; Nesper, R.; Fjellvåg, H.; Norby, P. J. Phys. Chem. C 2008, 112, 13134.
[19] Ananth, M. V.; Pethkar, S.; Dakshinamurthi, K. J. Power Sources 1998, 75, 278.
[20] Portehault, D.; Cassaignon, S.; Baudrin, E.; Jolivet, J. P. Chem. Mater. 2007, 19, 5410.
[21] Hou, J.; Liu, L.; Li, Y.; Mao, M.; Lv, H.; Zhao, X. Environ. Sci. Technol. 2013, 47, 13730.
[22] Tang, W.; Shan, X.; Li, S.; Liu, H.; Wu, X.; Chen, Y. Mater. Lett. 2014, 132, 317.
[23] Carno, J.; Ferrandon, M.; Bjrnbom, E.; Jaras, S. Appl. Catal. A:Gen. 1997, 155, 265.
[24] Ivanova, S.; Petit, C.; Pitchon, V. Appl. Catal. A:Gen. 2007, 267, 191.
[25] Gac, W. Appl. Catal. B:Environ. 2007, 75, 107.
[26] Zhang, X.; Sun, X.; Zhang, H.; Li, C.; Ma, Y. Electrochim. Acta 2014, 132, 315.
[27] Chai, C.; Liu, A.; Lv, Y.; Mu, J.; Zhang, X.; Che, H. Mater. Lett. 2017, 196, 308.
[28] Wan, C.; Wang, L.; Shen, S.; Zhu, X. Acta Chim. Sinica 2009, 67, 1559(in Chinese). (万传云, 王利军, 沈绍典, 朱贤, 化学学报, 2009, 67, 1559.)
[29] Dong, S.; Chen, X.; Gu, L.; Zhou, X.; Li, L.; Liu, Z.; Han, P.; Xu, H.; Yao, J.; Wang, H.; Zhang, X.; Shang, C.; Cui, G.; Chen, L. Energy Environ. Sci. 2011, 4, 3502.
/
| 〈 |
|
〉 |