

Synthesis of Tetrahydropyrrolo/indolo[1,2-a]pyrazines by Enantioselective Hydrogenation of Heterocyclic Imines
Received date: 2017-11-02
Online published: 2018-01-09
Supported by
Project supported by the National Natural Science Foundation of China (Nos. 21532006, 21690074), the Chinese Academy of Sciences (No. QYZDJ-SSW-SLH035) and Dalian Bureau of Science and Technology (No. 2016RD07).
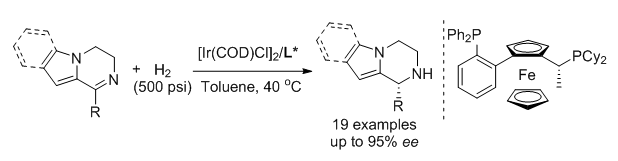
1,2,3,4-Tetrahydropyrrolo[1,2-a]pyrazines are an important motif due to their biological activities and widely existing in natural products. Notably, the substituent and the absolute configuration are important for the medicinal efficacy. Thus, the synthesis of chiral tetrahydropyrrolo[1,2-a]pyrazines has attracted much attention of scientists. Most synthetic methods utilized chiral starting materials or auxiliaries. Kinetic resolution was an alternative way to give chiral tetrahydropyrrolo[1,2-a]pyrazines. The first cata-lytic asymmetric synthetic method was developed in 2011 by Li and Antilla through a chiral phosphoric acid-catalyzed asymmetric intramolecular aza-Friedel-Crafts reaction of aldehydes with N-aminoethylpyrroles in high enantiocontrol level. Subsequently, the sequential aerobic oxidation-asymmetric intramolecular aza-Friedel-Crafts reaction between N-aminoethylpyrroles and benzyl alcohols for the synthesis of tetrahydropyrrolo[1,2-a]pyrazines was realized using chiral bifunctional heterogeneous materials composed of Au/Pd nanoparticles and chiral phosphoric acids. The asymmetric hydrogenation as an efficient way has been successfully applied to synthesize the kind of chiral amines. In 2012, Our group achieved the asymmetric hydrogenation of 1-substituted pyrrolo[1,2-a]pyrazines via a substrate activation strategy. Recently, we reported the direct asymmetric hydrogenation of 3-substituted pyrrolo[1,2-a]pyrazines in up to 96% ee values. Considering their impressive significance, herein, we successfully hydrogenated 3,4-dihydropyrrolo[1,2-a]pyrazines and 3,4-dihydroindolo[1,2-a]pyrazines with up to 99% yield and 95% ee. The reaction features mild condition, high enantioselectivity and high atom-economy. The typical procedure for asymmetric hydrogenation is as follows:A mixture of[Ir(COD)Cl]2 (3.0 mg, 0.0045 mmol) and the ligand Cy-WalPhos (6.6 mg, 0.0099 mmol) was stirred in toluene (1.0 mL) at room temperature for 5 min in the glove box. Then the solution was transferred to the vial containing the substrate 3,4-dihydropyrrolo[1,2-a]pyrazines (0.3 mmol) together with toluene (2.0 mL). The vial was taken to an autoclave and the hydrogenation was conducted at 40℃ as well as at a hydrogen pressure of 500 psi for 48 h. After carefully releasing the hydrogen, the autoclave was opened and the toluene was evaporated in vacuo. The residue was purified by column chromatography to afford the corresponding chiral tetrahydropyrrolo[1,2-a]pyrazines.
Hu Shu-Boa , Chen Mu-Wang , Zhai Xiao-Yong , Zhou Yong-Gui . Synthesis of Tetrahydropyrrolo/indolo[1,2-a]pyrazines by Enantioselective Hydrogenation of Heterocyclic Imines[J]. Acta Chimica Sinica, 2018 , 76(2) : 103 -106 . DOI: 10.6023/A17110476
[1] (a) Al-Mourabit, A.; Zancanella, M. A.; Tilvi, S.; Romo, D. Nat. Prod. Rep. 2011, 28, 1229;
(b) Papeo, G.; Gómez-Zurita Frau, M. A.; Borghi, D.; Varasi, M. Tetrahedron Lett. 2005, 46, 8635.
[2] (a) Peresada, V. P.; Medvedev, O. S.; Likhosherstov, A. M.; Skoldinov, A. P. Khim. Farm. Zh. 1987, 21, 1054;
(b) Seredenin, S. B.; Voronina, T. A.; Likhosherstov, A. M.; Peresada, Y. P.; Molodavkin, G. M.; Halikas, J. A. US 5378846, 1995;
(c) Seredenin, S. B.; Voronina, T. A.; Beshimov, A.; Peresada, V. P.; Likhosherstov, A. M. RU 2099055, 1997;
(d) Negoro, T.; Murata, M.; Ueda, S.; Fujitani, B.; Ono, Y.; Kuromiya, A.; Komiya, M.; Suzuki, K.; Matsumoto, J.-I. J. Med. Chem. 1998, 41, 4118;
(e) Likhosherstov, A. M.; Filippova, O. V.; Peresada, V. P.; Kryzhanovskii, S. A.; Vititnova, M. B.; Kaverina, N. V.; Reznikov, K. M. Pharm. Chem. J. 2003, 37, 6;
(f) Merla, B.; Christoph, T.; Oberboersch, S.; Schiene, K.; Bahrenberg, G.; Frank, R.; Kuehnert, S.; Schroeder, W. WO 2008046582, 2008[Chem. Abstr. 2008, 148, 472076];
(g) Henrich, M.; Weil, T.; Müller, S.; Nagel, J.; Gravius, A.; Kauss, V.; Zemribo, R.; Erdmane, E. WO 2009095254, 2009[Chem. Abstr. 2009, 151, 221195];
(h) Gahman, T. C.; Zhao, C.; Lang, H.; Massari, M. E. US 20090062253, 2009[Chem. Abstr. 2009, 150, 283093];
(i) Zhu, B.; Marinelli, B. A.; Goldschmidt, R.; Foleno, B. D.; Hilliard, J. J.; Bush, K.; Macielag, M. Bioorg. Med. Chem. Lett. 2009, 19, 4933.
[3] (a) Li, G.; Rowland, G. B.; Rowland, E. B.; Antilla, J. C. Org. Lett. 2007, 9, 4065;
(b) Gualandi, A.; Cerisoli, L.; Monari, M.; Savoia, D. Synthesis 2011, 909;
(c) Bhowmik, S.; Kumar, A. K. S.; Batra, S. Tetrahedron Lett. 2013, 54, 2251.
[4] Kreituss, I.; Chen, K.-Y.; Eitel, S. H.; Adam, J.-M.; Wuitschik, G.; Fettes, A.; Bode, J. W. Angew. Chem., Int. Ed. 2016, 55, 1553.
[5] He, Y.; Lin, M.; Li, Z.; Liang, X.; Li, G.; Antilla, J. C. Org. Lett. 2011, 13, 4490.
[6] Cheng, H.-G.; Miguélez, J.; Miyamura, H.; Yoo, W.-J.; Kobayashi, S. Chem. Sci. 2017, 8, 1356.
[7] For some examples, see:(a) Glorius, F. Org. Biomol. Chem. 2005, 3, 4171;
(b) Lu, S.-M.; Han, X.-W.; Zhou, Y.-G. Chin. J. Org. Chem. 2005, 25, 634(in Chinese). (卢胜梅, 韩秀文, 周永贵, 有机化学, 2005, 25, 634.)
(c) Zhou, Y.-G. Acc. Chem. Res. 2007, 40, 1357;
(d) Kuwano, R. Heterocycles 2008, 76, 909;
(e) Xie, J.; Zhou, Q. Acta Chim. Sinica 2012, 70, 1427(in Chinese). (谢建华, 周其林, 化学学报, 2012, 70, 1427.)
(f) Yu, Z.; Jin, W.; Jiang, Q. Angew. Chem. Int. Ed. 2012, 51, 6060;
(g) Wang, D.-S.; Chen, Q.-A.; Lu, S.-M.; Zhou, Y.-G. Chem. Rev. 2012, 112, 2557;
(h) He, Y.-M.; Song, F.-T.; Fan, Q.-H. Top. Curr. Chem. 2014, 343, 145;
(i) Nagano, T.; Iimuro, A.; Yamaji, K.; Kita, Y.; Mashima, K. Heterocycles 2014, 88, 103;
(j) Yuan, Q.; Zhang, W. Chin. J. Org. Chem. 2016, 36, 274(in Chinese). (袁乾家, 张万斌, 有机化学, 2016, 36, 274.)
(k) Chen, Z.-P.; Zhou, Y.-G. Synthesis 2016, 48, 1769;
(l) Zhang, Z.; Butt, N. A.; Zhang, W. Chem. Rev. 2016, 116, 14769;
(m) Wang, H.; Zheng, Y.; Pan, Z.; Fu, H.; Ling, F.; Zhong, W. Chin. J. Org. Chem. 2017, 37, 301(in Chinese). (王辉, 郑亿, 潘振涛, 傅鸿樑, 凌飞, 钟为慧, 有机化学, 2017, 37, 301.);
(n) Liu, Y.; Du, H. Acta Chim. Sinica 2014, 72, 771(in Chinese). (刘勇兵, 杜海峰, 化学学报, 2014, 72, 771.);
(o) Wang, D.; Hou, C.; Chen, L.; Liu, X.; An, Q.; Hu, X. Chin. J. Org. Chem. 2013, 33, 1355. (in Chinese). (王东, 侯传金, 陈丽凤, 刘小宁, 安庆大, 胡向平, 有机化学, 2013, 33, 1355.)
(p) Li, C.; Xiao, J. J. Am. Chem. Soc. 2008, 130, 13208;
(q) Ding, Z.-Y.; Chen, F.; Qin, J.; He, Y.-M.; Fan, Q.-H. Angew. Chem., Int. Ed. 2012, 51, 5706;
(r) Guo, C.; Sun, D.-W.; Yang, S.; Mao, S.-J.; Xu, X.-H.; Zhu, S.-F.; Zhou, Q.-L. J. Am. Chem. Soc. 2015, 137, 90;
(s) Zhou, H.; Liu, Y.; Yang, S. Angew. Chem., Int. Ed. 2017, 56, 2725.
[8] Huang, W.-X.; Yu, C.-B.; Shi, L.; Zhou, Y.-G. Org. Lett. 2014, 16, 3324.
[9] Hu, S.-B.; Chen, Z.-P.; Song, B.; Wang, J.; Zhou, Y.-G. Adv. Synth. Catal. 2017, 359, 2762.
[10] (a) Xiao, D.; Zhang, X. Angew. Chem., Int. Ed. 2001, 40, 3425;
(b) Wang, D.-W.; Wang, X.-B.; Wang, D.-S.; Lu, S.-M.; Zhou, Y.-G.; Li, Y.-X. J. Org. Chem. 2009, 74, 2780;
(c) Ji, Y.; Shi, L.; Chen, M.-W.; Feng, G.-S.; Zhou, Y.-G. J. Am. Chem. Soc. 2015, 137, 10496.
/
| 〈 |
|
〉 |