

A New Decarboxylation/Methylation Process of Cinnamic Acids
Received date: 2017-11-30
Online published: 2018-01-09
Supported by
Project supported by the National Natural Science Foundation of China (Nos. 21402068, 21574060, 21472177).
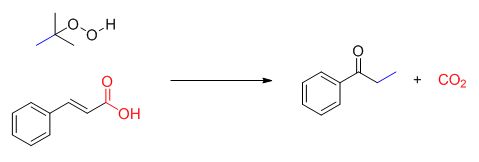
Decarboxylation reactions have been widely explored in recent ten years, and decarboxylative cascade reaction of cinnamic acids has also attracted much attention. Generally, this class of reaction includes two processes:radical addition and decarboxylation. The result of reaction can introduce kinds of functional groups on the benzene ring, such as halogen atom, nitro-group and trifluoromethyl. Following the continuous studies of our group on oxidative cascade reaction, especially the oxidative reaction in the absence of metal, herein we disclosed a decarboxylative oxidative cascade reaction of cinnamic acids under metal-free conditions. We employed K2CO3 as base, tert-butyl hydroperoxide (TBHP) as oxidant and DMSO/H2O as co-solvent, and cinnamic acids could be converted to propiophenone derivatives in moderate yield. To get an insight into the mechanism of this process, several controlled experiments were conducted. First, DMSO-d6 was employed under the standard conditions and no d-methyl was detected in the 1H NMR spectrum of product. It demonstrated that the methyl of product was derived from tert-butyl hydroperoxide. Subsequently, the reaction was carried out under nitrogen and oxygen atmosphere, and it was found that higher reaction efficiency was obtained in N2. The result indicated that methyl radical was easily quenched by oxygen. On the basis of experiment results, we proposed a plausible mechanism. First, tert-butyl hydroperoxide yielded methyl radical, then the reaction underwent radical addition and decarboxylation to generate the desired product. The reaction featured that tert-butyl hydroperoxide was used as oxidant and methylation reagent in this process, and the reaction proceeded under metal-free and aqueous phase condition. Therefore, it met the requirement of green chemistry. Meanwhile, the operation of reaction was also simple, for example, to a DMSO/H2O (0.5 mL/0.5 mL) solution of cinnamic acids (0.2 mmol) were successively added K2CO3 (0.3 mmol), TBHP (0.8 mmol). The reaction mixture was stirred at 100 ℃. Upon the completion, the desired product was purified by silica gel column chromatography.
Ye Wenbo , Yan Zicong , Wan Changfeng , Hou Haoqing , Wang Zhiyong . A New Decarboxylation/Methylation Process of Cinnamic Acids[J]. Acta Chimica Sinica, 2018 , 76(2) : 99 -102 . DOI: 10.6023/A17110519
[1] (a) Goossen, L. J.; Thiel, W. R.; Rodriguez, N.; Linder, C.; Melzer, B. Adv. Synth. Catal. 2007, 349, 2241.
(b) Cornella, J.; Sanchez, C.; Banawa, D.; Larrosa, I. Chem. Commun. 2009, 7176.
(c) Lu, P. F.; Sanchez, C.; Cornella, J.; Larrosa, I. Org. Lett. 2009, 11, 5710.
[2] (a) Goossen, L. J.; Rodriguez, N.; Melzer, B.; Linder, C.; Deng, G.; Levy, L. M. J. Am. Chem. Soc. 2007, 129, 4824.
(b) Goossen, L. J.; Zimmermann, B.; Knauber, T. Angew. Chem., Int. Ed. 2008, 47, 7103.
(c) Zhou, Q. Q.; Liu, D.; Xiao, W. J.; Lu, L. Q. Acta Chim. Sinica 2017, 75, 110. (周泉泉, 刘丹, 肖文精, 陆良秋, 化学学报, 2017, 75, 110.)
(d) Yuan, J. W.; Yang, L. R.; Mao, P.; Qu, L. B. Org. Chem. Front. 2017, 4, 454.
[3] (a) Zhang, C.; Seidel, D. J. Am. Chem. Soc. 2010, 132, 1798.
(b) Bi, H. P.; Teng, Q. F.; Guan, M.; Chen, W. W.; Liang, Y. M.; Yao, X. J.; Li, C. J. J. Org. Chem. 2010, 75, 783.
(c) Lo, Vanessa, K. Y.; Wong, M. K.; Che, C. M. Org. Lett. 2008, 10, 517.
(d) Guan, B. C.; Xu, X. L.; Wang, H.; Li, X. N. Chin. J. Org. Chem. 2016, 36, 1564. (关保川, 许孝良, 王红, 李小年, 有机化学, 2016, 36, 1564.)
[4] (a) Borah, A. J.; Yan, G. B. Org. Biomol. Chem. 2015, 13, 8094.
(b) Li, Z.; Zheng, J.; Hu, W.; Li, J.; Wu, W.; Jiang, H. Org. Chem. Front. 2017, 4, 1363.
(c) Yu, J.; Mao, R.; Wang, Q.; Wu, J. Org. Chem. Front. 2017, 4, 617.
(d) Wu, Z. J.; Wang, J. Acta Chim. Sinica 2017, 75, 74. (吴自俊, 汪舰, 化学学报, 2017, 75, 74.)
(e) Li, Z.; Zheng, J.; Hu, W. G. Org. Chem. Front. 2017, 4, 1363.
[5] (a) Yin, J.; Li, Y.; Zhang, R.; Jin, K.; Duan, C. Synthesis-Structure 2014, 607.
(b) Shang, X. J.; Li, Z.; Liu, Z. Q. Tetrahedron Lett. 2015, 56, 233.
[6] (a) Telvekar, V. N.; Arote, N. D.; Herlekar, O. P. Synlett 2005, 2495.
(b) Telvekar, V. N.; Takale, B. S. Tetrahedron Lett. 2011, 52, 2394.
[7] (a) Rao, A. S.; Srinivas, P. V.; Babu, K. S.; Rao, J. M. Tetrahedron Lett. 2005, 46, 8141.
(b) Ramgopal, S.; Ramesh, K.; Chakradhar, A.; Reddy, N. M.; Rajanna, K. C. Tetrahedron Lett. 2007, 48, 4043.
(c) Rajanna, K. C.; Ramesh, K.; Ramgopal, S.; Shylaja, S.; Reddy, P. G.; Saiprakash, P. K. Green Sustainable Chem. 2011, 1, 132.
[8] (a) Rokade, B. V.; Prabhu, K. R. J. Org. Chem. 2014, 79, 8110.
(b) Guo, R.; Gui, Q.; Wang, D.; Tan, Z. Catal. Lett. 2014, 144, 1377.
[9] (a) Vinokurov, N.; Michrowska, A.; Szmigielska, A.; Drzazga, Z.; Wójciuk, G.; Demchuk, O. M.; Grela, K.; Pietrusiewicz, K. M.; Butenschön, H. Adv. Synth. Catal. 2006, 348, 931.
(b) Al-Maksoud, W.; Mesnager, J.; Jaber, F.; Pinel, C.; Djakovitch, L. J. Organomet. Chem. 2009, 694, 3222.
(c) Evano, G.; Tadiparthiand, K.; Couty, F.; Chem. Commun. 2011, 47, 179.
(d) Jouvin, K.; Coste, A.; Bayle, A.; Legrand, F.; Karthikeyan, G.; Tadiparthiand, K.; Evano, G. Organometallics 2012, 31, 7933.
[10] (a) Yang, Y.; Yao, J.; Zhang, Y. Org. Lett. 2013, 15, 3206.
(b) Yang, Y.; Chen, L.; Zhang, Z.; Zhang, Y. Org. Lett. 2011, 13, 1342.
[11] Rong, G. W.; Liu, D. F.; Lu, L. H.; Yan, H.; Zheng, Y.; Chen, J.; Mao, J. C. Tetrahedron 2014, 70, 5033.
[12] Ji, J.; Liu, P.; Sun, P. P. Chem. Commun. 2015, 51, 7546.
/
| 〈 |
|
〉 |