

Application of Photochemical Rearrangement of Santonin in Total Synthesis of Complex Natural Terpenoids
Received date: 2017-12-11
Online published: 2018-01-26
Supported by
Project supported by the National Natural Science Foundation of China (Nos. 21422203, 21772044), the National Young Top-Notch Talent Support Program and the Fundamental Research Funds for the Central Universities.
Terpenoids represent one of the largest and most diverse classes of secondary metabolites and widely exist in nature. Among them, sesquiterpene lactones are ubiquitous in a variety of medicinal plants, which are the main active ingredients of many traditional Chinese herbal medicines. However, it is extremely challenging to accomplish the total synthesis of these natural compounds. Photochemical rearrangement of santonin is an effective strategy to construct the guaianolide skeleton. Furthermore, as a renewable natural resource, santonin was extensively used in natural product total synthesis, especially complex terpenoids. In this review, a brief overview of application of photochemical rearrangement of santonin in total synthesis of natural terpenoids is presented, which mainly includes:(1) the synthesis of sesquiterpene and its oligomers, and (2) the core structure construction of some diterpenoids.
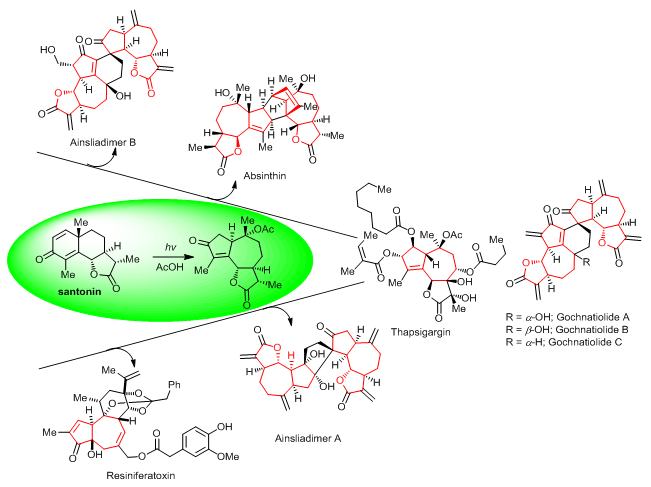
Yang Baochao , Gao Shuanhu . Application of Photochemical Rearrangement of Santonin in Total Synthesis of Complex Natural Terpenoids[J]. Acta Chimica Sinica, 2018 , 76(3) : 161 -167 . DOI: 10.6023/A17120537
[1] Maimone, T. J.; Baran, P. S. Nat. Chem. Biol. 2007, 3, 396.
[2] Zhang, D.; Xie, X.; Feng, J. S.; Wen, H. L. Chin. J. Org. Chem. 2016, 36, 202. (张迪, 谢雄, 冯金生, 温鸿亮, 有机化学, 2016, 36, 202.)
[3] Zhou, W. S.; Huang, M. L. Acta Chim. Sinica 1956, 4, 73. (周维善, 黄鸣龙, 化学学报, 1956, 4, 73.)
[4] (a) Wang, J. G.; Zhu, X. X. Chin. Sci. Bull. 2017, 62, 1973. (王继刚, 朱晓新, 科学通报, 2017, 62, 1973.)
(b) Liu, D. L.; Zhang, W. B. Chin. Sci. Bull. 2017, 62, 1997. (刘德龙, 张万斌, 科学通报, 2017, 62, 1997.)
[5] Tang, P.; Wang, F. P. Chin. J. Org. Chem. 2013, 33, 458. (唐培, 王锋鹏, 有机化学, 2013, 33, 458.)
[6] (a) Cherney, E. C.; Baran, P. S. Isr. J. Chem. 2011, 51, 391. For selected examples of terpenoids isolation, see:
(b) Chen, D. W.; Liu, D.; Shen, S.; Cheng, W.; Lin, W. H. Chin. J. Chem. 2012, 30, 1459.
(c) Wang, X. J.; Yu, D. Q.; Yu, S. S. Chin. J. Chem. 2014, 32, 1007.
(d) Cao, T. W.; He, K.; Geng, C. A.; Zhang, X. M.; Zhou, J.; Chen, J. J. Chin. J. Org. Chem. 2016, 36, 2216. (曹团武, 何康, 耿长安, 张雪梅, 周俊, 陈纪军, 有机化学, 2016, 36, 2216.) For selected examples of terpenoids synthesis, see:
(e) Yue, G. Z.; Huang, X.; Liu, B. Chin. J. Org. Chem. 2013, 33, 1167. (乐贵洲, 黄轩, 刘波, 有机化学, 2013, 33, 1167.)
(f) Lu, Y. P.; Qi, W. P.; Weng, G. D.; Zhang, X. X. Chin. J. Org. Chem. 2015, 35, 1407. (卢勇平, 祁伟鹏, 翁国栋, 张兴贤, 有机化学, 2015, 35, 1407.)
(g) Sun, Y.; Meng, Z. C.; Chen, P. X.; Zhang, D. L.; Baunach, M.; Hertweck, C.; Li, A. Org. Chem. Front. 2016, 3, 368.
[7] (a) Adekenov, S. M. Euras. Chem.-Technol. J. 2013, 15, 163.
(b) Zhou, W. S.; Yin, Z. H.; Huang, M. L. Acta Chim. Sinica 1964, 30, 32. (周维善, 殷芝华, 黄鸣龙, 化学学报, 1964, 30, 32.)
[8] (a) Bach, T.; Hehn, J. P. Angew. Chem. Int. Ed. 2011, 50, 1000.
(b) Wan, C. Y.; Deng, J.; Liu, H.; Bian, M.; Li, A. Sci. China Chem. 2014, 57, 926. Recently Wang et al. achieve the total synthesis of (+)-Chinensiolide B from photochemical rearrangement of santonin, for details see:
(c) Zhang, L. Q.; Dai, X. L.; Tao, L. Z.; Xie, C. S.; Zhang, M.; Wang, M. Chin. J. Chem. 2017, 35, 1284.
[9] Trommsdorff, H. Ann. Chem. Pharm. 1834, 11, 190.
[10] Barton, D. H. R.; de Mayo, P.; Shafiq, M. J. Chem. Soc. 1957, 929.
[11] For selected examples, see:(a) White, E. H.; Marx, J. N. J. Am. Chem. Soc. 1967, 89, 5511.
(b) Marx, J. N.; White, E. H. Tetrahedron 1969, 25, 2117.
(c) Edgar, M. T.; Greene, A. E.; Crabbe, P. J. Org. Chem. 1979, 44, 159.
(d) Greene, A. E. J. Am. Chem. Soc. 1980, 102, 5337.
(e) Greene, A. E.; Edgar, M. T. J. Org. Chem. 1989, 54, 1468.
(f) Delair, P.; Kann, N.; Greene, A. E. J. Chem. Soc. Perkin Trans. 11994, 1651.
(g) Sun, L. D.; Tu, Y. Q. Tetrahedron Lett. 1998, 39, 7935.
(h) Blay, G.; Bargues, V.; Cardona, L.; Collado, A. M.; García, B.; Muñoz, M. C.; Pedro, J. R. J. Org. Chem. 2000, 65, 2138.
(i) Blay, G.; Bargues, V.; Cardona, L.; García, B.; Pedro, J. R. J. Org. Chem. 2000, 65, 6703.
(j) Blay, G.; Bargues, V.; Cardona, L.; García, B.; Pedro, J. R. Tetrahedron 2001, 57, 9719.
(k) Blay, G.; Cardona, L.; García, B.; Lahoz, L.; Pedro, J. R. J. Org. Chem. 2001, 66, 7700.
(l) Xia, W. J.; Li, D. R.; Tu, Y. Q. Synth. Commun. 2001, 31, 1613.
(m) Xia, W. J.; Sun, L. D.; Shi, L.; Zhang, S. Y.; Tu, Y. Q. Chin. J. Chem. 2004, 22, 377.
(n) Makiyi, E. F.; Frade, R. F. M.; Lebl, T.; Jaffray, E. G.; Cobb, S. E.; Harvey, A. L.; Slawin, A. M. Z.; Hay, R. T.; Westwood, N. J. Eur. J. Org. Chem. 2009, 2009, 5711.
(o) Dai, X. L.; Tang, J.; Zhang, L. Q.; Tao, L. Z.; Ouyang, Z.; Wang, M. Chin. J. Org. Chem. 2015, 35, 2142. (戴笑丽, 汤建, 章凌琼, 陶连芝, 欧阳臻, 王民, 有机化学, 2015, 35, 2142.)
[12] (a) Natarajan, A.; Tsai, C. K.; Khan, S. I.; McCarren, P.; Houk, K. N.; Garcia-Garibay, M. A. J. Am. Chem. Soc. 2007, 129, 9846.
(b) Commins, P.; Natarajan, A.; Tsai, C. K.; Khan, S. I.; Nath, N. K.; Naumov, P.; Garcia-Garibay, M. A. Cryst. Growth Des. 2015, 15, 1983.
(c) Chen, X.; Tian, G. J.; Rinkevicius, Z.; Vahtras, O.; Cao, Z. X.; Ågren, H.; Luo, Y. Chem. Phys. 2012, 405, 40.
(d) Chen, X.; Rinkevicius, Z.; Luo, Y.; Ågren, H.; Cao, Z. X. ChemPhysChem 2012, 13, 353.
[13] (a) Herout, V.; Sorm, F. Collect. Czech. Chem. Commun. 1953, 18, 854.
(b) Herout, V.; Sorm, F. Collect. Czech. Chem. Commun. 1954, 19, 792.
(c) Novotny, L.; Herout, V.; Sorm, F. Collect. Czech. Chem. Commun. 1960, 25, 1492 and references therein.
[14] Zhang, W. H.; Luo, S. J.; Fang, F.; Chen, Q. S.; Hu, H. W.; Jia, X. S.; Zhai, H. B. J. Am. Chem. Soc. 2005, 127, 18.
[15] Vokac, K.; Samek, Z.; Herout, V.; Sorm, F. Tetrahedron Lett. 1968, 9, 3855.
[16] Li, C.; Yu, X. L.; Lei, X. G. Org. Lett. 2010, 12, 4284.
[17] Wu, Z. J.; Xu, X. K.; Shen, Y. H.; Su, J.; Tian, J. M.; Liang, S.; Li, H. L.; Liu, R. H.; Zhang, W. D. Org. Lett. 2008, 10, 2397.
[18] Doyle, A. G.; Jacobsen, E. N. Chem. Rev. 2007, 107, 5713.
[19] (a) Li, C.; Dian, L. Y.; Zhang, W. D.; Lei, X. G. J. Am. Chem. Soc. 2012, 134, 12414.
(b) Li, C.; Dong, T.; Dian, L. Y.; Zhang, W. D.; Lei, X. G. Chem. Sci. 2013, 4, 1163.
(c) Li, C.; Lei, X. G. J. Org. Chem. 2014, 79, 3289.
(d) Li, C.; Dong, T.; Li, Q.; Lei, X. G. Angew. Chem. Int. Ed. 2014, 53, 12111.
[20] Xia, D. L.; Du, Y.; Yi, Z.; Song, H.; Qin, Y. Chem. Eur. J. 2013, 19, 4423.
[21] (a) Bohlmann, F.; Ahmed, M.; Jakupovic, J.; King, R. M.; Robinson, H. Phytochemistry 1983, 22, 191.
(b) Bohlmann, F.; Zdero, C.; Hirschmann-Schmeda, G.; Jakupovic, J.; Dominguez, X. A.; King, R. M.; Robinson, H. Phytochemistry 1986, 25, 1175.
(c) Wang, Y.; Shen, Y. H.; Jin, H. Z.; Fu, J. J.; Hu, X. J.; Qin, J. J.; Liu, J. H.; Chen, M.; Yan, S. K.; Zhang, W. D. Org. Lett. 2008, 10, 5517.
[22] (a) Chu, H.; Smith, J. M.; Felding, J.; Baran, P. S. ACS Cent. Sci. 2017, 3, 47. For other related synthesis, see:
(b) Oliver, S. F.; Högenauer, K.; Simic, O.; Antonello, A.; Smith, M. D.; Ley, S. V. Angew. Chem. Int. Ed. 2003, 42, 5996.
(c) Ball, M.; Andrews, S. P.; Wierschem, F.; Cleator, E.; Smith, M. D.; Ley, S. V. Org. Lett. 2007, 9, 663.
(d) Chen, D. Z.; Evans, P. A. J. Am. Chem. Soc. 2017, 139, 6046.
[23] (a) Rasmussen, U.; Christensen, S. B. Acta Pharm. Suec. 1978, 15, 133.
(b) Smitt, U. W.; Christensen, S. B. Planta Med. 1991, 57, 196.
(c) Liu, H.; Jensen, K. G.; Tran, L. M.; Chen, M.; Zhai, L.; Olsen, C. E.; Søhoel, H.; Denmeade, S. R.; Isaacs, J. T.; Christensen, S. B. Phytochemistry 2006, 67, 2651.
[24] Thastrup, O.; Cullen, P. J.; Drøbak, B. K.; Hanley, M. R.; Dawson, A. P. Proc. Natl. Acad. Sci. U. S. A. 1990, 87, 2466.
[25] Hergenhahn, M.; Adolf, W.; Hecker, E. Tetrahedron Lett. 1975, 16, 1595.
[26] Wender, P. A.; Jesudason, C. D.; Nakahira, H.; Tamura, N.; Tebbe, A. L.; Ueno, Y. J. Am. Chem. Soc. 1997, 119, 12976.
[27] Jackson, S. R.; Johnson, M. G.; Mikami, M.; Shiokawa, S.; Carreira, E. M. Angew. Chem., Int. Ed. 2001, 40, 2694.
[28] Flekhter, O. B.; Nigmatullina, L. R.; Baltina, L. A.; Karachurina, L. T.; Galin, F. Z.; Zarudii, F. S.; Tolstikov, G. A.; Boreko, E. I.; Pavlova, N. I.; Nikolaeva, S. N.; Savinova, O. V. Pharm. Chem. J. 2002, 36, 484.
[29] Al-Dabbas, M. M.; Hashinaga, F.; Abdelgaleil, S. A. M.; Suganuma, K.; Akiyama, H. H. J. Ethnopharmacol. 2005, 97, 237.
[30] Shi, Y. S.; Liu, Y. B.; Ma, S. G.; Li, Y.; Qu, J.; Li, L.; Yuan, S. P.; Hou, Q.; Li, Y. H.; Jiang, J. D.; Yu, S. S. J. Nat. Prod. 2015, 78, 1526.
[31] Li, D. L.; Zheng, X.; Chen, Y. C.; Jiang, S.; Zhang, Y.; Zhang, W. M.; Wang, H. Q.; Du, Z. Y.; Zhang, K. Arch. Pharm. Res. 2016, 39, 51.
[32] Komaki, H.; Tanaka, Y.; Yazawa, K.; Takagi, H.; Ando, A.; Nagata, Y.; Mikami, Y. J. Antibiot. 2000, 53, 75.
/
| 〈 |
|
〉 |