

Synthesis of Coin-like Vanadium Disulfide and Its Sodium Storage Performance
Received date: 2017-12-06
Online published: 2018-03-22
Supported by
Project supported by the National Key R&D Program (No. 2016YFB0901502), National Natural Science Foundation of China (Nos. 51771094, 51371100) and 111 Project (No. B12015).
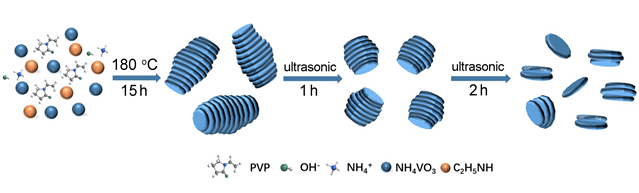
Sodium ion batteries (SIBs) have become one of candidates for post-lithium batteries due to the rich sodium resources and the similar physico-chemical properties between sodium and lithium, while the larger sodium ion radius affects the kinetic properties and ion mobility of the sodium ion batteries system, so finding the right electrode material has become the key to develop SIBs. Vanadium Disulfide (VS2) as a typical family member of transition metal chalcogenides (TMCs) has the graphene-like layered structure and excellent electrical conductivity, which provides sufficient space for the storage of sodium ions and ensures its high performance as anode for SIBs. In this work, we used the combination of hydrothermal method and ultrasonic stripping method to prepared three different Coin-like VS2 (VS2-Long, VS2-Middle, and VS2-Short) for sodium storage research. The results show that Coin-like VS2-Short (VS2-S) with the lowest stacking degree can expose more active sites and has a more stable structure so that it has a high capacity of 410 mAh·g-1 after 300 cycles at 100 mA·g-1 and a high rate capability of 333 mAh·g-1 even at 2000 mA·g-1. In addition, we also studied the mechanism of vanadium disulfide as electrode material of sodium ion batteries by using the ex-situ X-ray diffraction (XRD) and transmission electron microscopy (TEM). During discharge process, sodium ion was inserted into the layer of VS2 resulting in NaxVS2 at the voltage of 2.5~1.0 V, and then, NaxVS2 convert to sodium sulfide and vanadium between the voltage of 1.0~0.2 V, on the opposite charging process, sodium sulfide with vanadium will convert to NaxVS2 firstly and then vanadium disulfide will appeared again with the sodium ion deserted from the NaxVS2. This means that vanadium disulfide appears to be an insertion-conversion mechanism between 0.2~2.5 V.
Li Pan , Liu Jian , Sun Weiyi , Tao Zhanliang , Chen Jun . Synthesis of Coin-like Vanadium Disulfide and Its Sodium Storage Performance[J]. Acta Chimica Sinica, 2018 , 76(4) : 286 -291 . DOI: 10.6023/A17120533
[1] Xiang, X. D.; Zhang, K.; Chen, J. Adv. Mater. 2015, 27, 5343.
[2] Dunn, B.; Kamath, H.; Tarascon, J. M. Science 2011, 334, 928.
[3] Francisco, D. G.; Andreas, S.; Oriol, G. B. Renew. Sust. Energy Rev. 2012, 16, 2154.
[4] Chen, H. S.; Cong, T. N.; Yang, W. Prog. Nat. Sci. 2009, 19, 291.
[5] Nagelberg, A. S.; Worrell, W. L. J. Solid State Chem. 1979, 29, 345.
[6] Shacklette, L. W.; Jow, T. R.; Townsend, L. J. Electrochem. Soc. 1985, 135, 2669.
[7] Li, H.; Wu, C.; Wu, F.; Bai, Y. Acta Chim. Sinica 2014, 72, 21. (李慧, 吴川, 吴锋, 白莹, 化学学报, 2014, 72, 21.)
[8] Xiang, X. D.; Lu, Y. Y.; Chen, J. Acta Chim. Sinica 2017, 75, 154. (向兴德, 卢艳莹, 陈军, 化学学报, 2017, 75, 154.)
[9] Lin, X. Y.; Wang, Y.; Chen, J. Acta Chim. Sinica 2017, 75, 979. (林潇羽, 王璟, 化学学报, 2017, 75, 979.)
[10] Tan, C. L.; Lai, Z. C.; Zhang, H. Adv. Mater. 2017, 29, 1701392.
[11] Liu, Z. M.; Lu, T. C.; Song, T.; Ungyu, P. Energy Environ. Sci. 2017, 10, 1576.
[12] Lu, Y.; Zhao, Q.; Zhang, N.; Lei, K.; Li, F.; Chen, J. Adv. Funct. Mater. 2016, 26, 911.
[13] Liu, X.; Zhang, K.; Lei, K.; Lei, K.; Li, F.; Tao, Z. L.; Chen, J. Nano Res. 2016, 9(1), 198.
[14] Wang, Q. H.; Kourosh, K. Z.; Andras, K.; Jonathan, N.; Michael, S. Nat. Nanotechnol. 2012, 7, 699.
[15] Rout, C. S.; Kim, B. H.; Xu, X.; Yang, J.; Jeong, H. Y.; Odkhuu, D.; Park, N.; Cho, J.; Shin, H. S. J. Am. Chem. Soc. 2013, 135, 8720.
[16] Chang, K.; Chen, W. X. Chem. Commun. 2011, 47, 4252.
[17] Li, Y.; Wang, H.; Xie, L.; Liang, Y.; Hong, G.; Dai, H. J. Am. Chem. Soc. 2011, 133, 7296.
[18] Feng, J.; Peng, L.; Wu, C.; Sun, X.; Hu, S.; Lin, C.; Dai, J.; Yang, J.; Xie, Y. Adv. Mater. 2012, 24, 1969.
[19] Feng, J.; Sun, X.; Wu, C.; Peng, L.; Lin, C.; Hu, S.; Yang, J.; Xie, Y. J. Am. Chem. Soc. 2011, 133, 17832.
[20] Fang, W. Y.; Zhao, H. B.; Xie, Y. P.; Fang, J. H.; Xu, J. Q.; Chen, Z. W. ACS Appl. Mater. Interfaces 2015, 7, 13044.
[21] He, P.; Yan, M. Y.; Zhang, G. B.; Sun, R. M.; An, Q. Y.; Mai, L. Q. Adv. Energy Mater. 2017, 7, 1601920.
[22] Sun, R. M.; Wei, Q. L.; Sheng, J. Z.; Shi, C. W.; An, Q. Y.; Liu, S. J.; Mai, L. Q. Nano Energy 2017, 35, 396.
[23] Chandra, S. R.; Ruchita, K.; Dattatray, J. L. Eur. J. Inorg. Chem. 2014, 5331.
[24] Liu, X.; Shuai, H. L.; Huang, K. J. Anal. Methods 2015, 7, 8277.
[25] Rout, C. S.; Kim, B. H.; Xu, X.; Yang, J.; Jeong, H. Y.; Odkhuu, D.; Park, N.; Cho, J.; Shin, H. S. J. Am. Chem. Soc. 2013, 135, 8720.
[26] Li, Y.; Liang, Y.; Hernandez, F. C. R.; Yoo, H. D.; An, Q.; Yao, Y. Nano Energy 2015, 15, 453.
[27] Zhang, S. S. J. Mater. Chem. A 2015, 3, 7689.
[28] Qu, B.; Ma, C.; Ji, G.; Xu, C.; Xu, J.; Meng, Y. S.; Wang, T.; Lee, J. Y. Adv. Mater. 2014, 26, 3854.
[29] Hu, Z.; Wang, L. X.; Zhang, K.; Wang, J. B.; Cheng, F. Y.; Tao, Z. L.; Chen, J. Angew. Chem., Int. Ed. 2014, 53, 12794.
[30] Yang, C. H.; Ou, X.; Xiong, X. H.; Zheng, F. H.; Hu, R. Z.; Chen, Y.; Liu, M. L.; Huang, K. Energy Environ. Sci. 2017, 10, 107.
/
| 〈 |
|
〉 |