

Catalytic Oxidation of Arsenic in Water by Silver Nanoparticles
Received date: 2018-02-09
Online published: 2018-03-26
Supported by
Project supported by the National Key R&D Program of China (No. 2016YFA0203102), and the National Natural Science Foundation of China (Nos. 21337004, 21620102008).
With the development of nanoscience and nanotechnology, nanomaterials have been applied in many areas including environments. Silver nanoparticles (AgNPs) are being widely used in drinking water disinfection due to their excellent bactericidal performance. As the bactericide, AgNPs could minimize or eliminate bacteria exceeding standards and water treatment membrane fouling. Arsenic contamination, especially in the underground water, has gained great attention from the environmental science community, demanding effective methods to eliminate or remove more acutely toxic inorganic species[i.e., As(Ⅲ) and As(V)]. Given the good photocatalytic activity, AgNPs could have an impact on the transformaiton of As(Ⅲ) and As(V). In this study, high performance liquid chromatography (HPLC) coupled with inductively coupled plasma mass spectrometer (HPLC-ICP/MS) were used to investigate the effects of some environmental relative factors like pH, natural organic matter, cation ions (e.g., Ca2+), and the intrinsic properties of AgNPs like size and coatings, on the conversion of the two main inorganic arsenic[As(Ⅲ) and As(V)] in the aqueous solution in the presence of AgNPs. It was found that AgNPs showed no physical adsorption for As(Ⅲ), while resulted in significant catalytic oxidation of As(Ⅲ) into As(V). Moreover, environmental factors including pH, sunlight, NOM, Ca2+, and properties of AgNPs (e.g., size, coating) showed significant effects on the catalytic oxidation of As(Ⅲ). The catalytic oxidation was also confirmed in the real environmental waters. Finally, the catalytic ability of AuNPs and AgNPs were compared to unveil the mechanism of catalytic oxidation of As(Ⅲ) by AgNPs. In addition to oxidation of superoxo or peroxo species formed due to activation of molecular oxygen by the electron transfer from negatively charged AgNPs, the redox potential of silver (φΘAg+/Ag0=0.80 V) mostly contributed to the transformation of As(Ⅲ) into As(V). Therefore, given the coexisting of As(Ⅲ) and AgNPs in the water treatment system, AgNPs could play dual function in both sterilization and detoxification of As(Ⅲ), which paved the novel way to effectively treat As contamination.
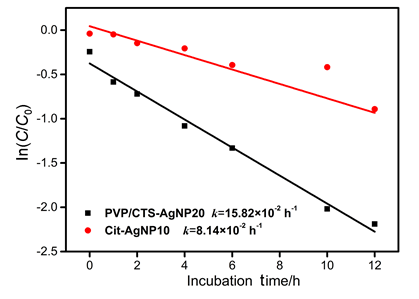
Key words: arsenic; silver nanoparticles; catalysis; environmental factors References
Guo Xiaoru , Yin Yongguang , Tan Zhiqiang , Liu Jingfu , Jiang Guibin . Catalytic Oxidation of Arsenic in Water by Silver Nanoparticles[J]. Acta Chimica Sinica, 2018 , 76(5) : 387 -392 . DOI: 10.6023/A18020067
[1] http://www.nanotechproject.org/cpi (accessed Feb 20, 2017).
[2] Cao, X. L.; Tang, M.; Liu, F.; Nie, Y. Y.; Zhao, C. S. Colloid Surface B 2010, 8, 555.
[3] Fendorf, S.; Michael, H. A.; Van Geen, A. Science 2010, 328, 1123.
[4] Yu, G. Q.; Sun, D. J.; Zheng, Y. Environ. Health Persp. 2007, 115, 636.
[5] Berg, M.; Tran, H. C.; Nguyen, T. C.; Pham, H. V.; Schertenleib, R.; Giger, W. Environ. Sci. Technol. 2001, 35, 2621.
[6] Karn, S. K. Environ. Pollut. 2015, 207, 434.
[7] He, J.; Charlet, L. J. Hydrol. 2013, 492, 79.
[8] Clancy, T. M.; Hayes, K. F.; Raskin, L. Environ. Sci. Technol. 2013, 47, 10799.
[9] Smedley, P. L.; Kinniburgh, D. G. Appl. Geochem. 2002, 17, 517.
[10] Sadee, B.; Foulkes, M. E.; Hill, S. J. J. Anal. At. Spectrom. 2015, 30, 102.
[11] Kumar, A. R.; Riyazuddin, P. Trends Anal. Chem. 2010, 29, 1212.
[12] Yang, K.; Xing, B. S. Chem. Rev. 2010, 110, 5989.
[13] Tan, K. B.; Vakili, M.; Hord, B. A.; Poh, P. E.; Abdullah, A. Z.; Salamatinia, B. Sep. Purif. Technol. 2015, 150, 229.
[14] Pena, M. E.; Korfiatis, G. P.; Patel, M.; Lippincott, L.; Meng, X. G. Water Res. 2005, 39, 2327.
[15] Hu, S.; Shi, Q. T.; Jing, C. Y. Environ. Sci. Technol. 2015, 49, 9707.
[16] Kanel, S. R.; Greneche, J. M.; Choi, H. Environ. Sci. Technol. 2006, 40, 2045.
[17] Gibert, O.; de Pablo, J.; Cortina, J. L.; Ayora, C. Environ. Geochem. Hlth. 2010, 32, 373.
[18] Xia, X. F.; Hua, Y. L.; Huang, X. Y.; Ling, L.; Zhang, W. X. Acta Chim. Sinica 2017, 75, 594. (夏雪芬, 滑熠龙, 黄潇月, 凌岚, 张伟贤, 化学学报, 2017, 75, 594.)
[19] Huang, X. Y.; Wang, W.; Ling L.; Zhang, W. X. Acta Chim. Sinica 2017, 75, 529. (黄潇月, 王伟, 凌岚, 张伟贤, 化学学报, 2017, 75, 529.)
[20] Xi, B. D.; Wang, X. W.; Liu, W. J.; Xia, X. F.; Li, D. S.; He, L. S.; Wang, H. M.; Sun, W. J.; Yang, T. X.; Tao, W. Sep. Sci. Technol. 2014, 49, 2642.
[21] Holt, B. D.; Heraty, L. J.; Sturchio, N. C. Environ. Pollut. 2001, 113, 263.
[22] Palau, J.; Jamin, P.; Badin, A.; Vanhecke, N.; Haerens, B.; Brouyere, S.; Hunkeler, D. Water Res. 2016, 92, 235.
[23] Rittmann, B. E.; Stilwell, D.; Garside, J. C.; Amy, G. L.; Spangenberg, C.; Kalinsky, A.; Akiyoshi, E. Water Res. 2002, 36, 3387.
[24] Mascolo, G.; Ciannarella, R.; Balest, L.; Lopez, A. J. Hazard. Mater. 2008, 152, 1138.
[25] Jeong, C. H.; Postigo, C.; Richardson, S. D.; Simmons, J. E.; Kimura, S. Y.; Marinas, B. J.; Barcelo, D.; Liang, P.; Wagner, E. D.; Plewa, M. J. Environ. Sci. Technol. 2015, 49, 13749.
[26] Yates, M. V.; Malley, J.; Rochelle, P.; Hoffman, R. J. Am. Water Works Ass. 2006, 98, 93.
[27] Mecha, C. A.; Pillay, V. L. J. Membrane Sci. 2014, 458, 149.
[28] Liga, M. V.; Bryant, E. L.; Colvin, V. L.; Li, Q. L. Water Res. 2011, 45, 535.
[29] Muthu, K.; Priya, S. Spectrochim. Acta A 2017, 179, 66.
[30] Baruah, B.; Gabriel, G. J.; Akbashev, M. J.; Booher, M. E. Langmuir 2013, 29, 4225.
[31] Joseph, S.; Mathew, B. J. Mol. Liq. 2015, 204, 184.
[32] Xu, R.; Wang, D. S.; Zhang, J. T.; Li, Y. D. Chem.-Asian. J. 2006, 1, 888.
[33] Morallon, E.; Arias-Pardilla, J.; Calo, J. M.; Cazorla-Amoros, D. Electrochim. Acta 2009, 54, 3996.
[34] Yu, S. J.; Yin, Y. G.; Liu, J. F. Environ. Sci. Proc. Impacts. 2013, 15, 78.
[35] Levard, C.; Hotze, E. M.; Lowry, G. V.; Brown, G. E. Environ. Sci. Technol. 2012, 46, 6900.
[36] Yin, Y. G.; Liu, J. F.; Jiang, G. B. ACS Nano 2012, 6, 7910.
[37] Kloster, N.; Brigante, M.; Zanini, G.; Avena, M. Colloid. Surface. A 2013, 427, 76.
[38] Wang, J.; Liu, J. J.; Guo, X. H.; Yan, L.; Lincoln, S. F. Front. Chem. Sci. Eng. 2016, 10, 432.
[39] Priya, D. B.; Asharani, I. V. J. Clust. Sci. 2017, 28, 1837.
[40] Chakraborty, I.; Pradeep, T. Chem. Rev. 2017, 117, 8208.
[41] Majdalawieh, A.; Kanan, M. C.; El-Kadri, O.; Kanan, S. M. J. Nanosci. Nanotechnol. 2014, 14, 4757.
[42] Okumura, M.; Haruta, M.; Kitagawa, Y.; Yamaguchia, K. Gold Bull. 2007, 40, 40.
[43] Ishida, T.; Nagaoka, M.; Akita, T.; Haruta, M. Chemistry 2008, 14, 8456.
[44] Tan, Z. Q.; Liu, J. F.; Yin, Y. G.; Shi, Q. T.; Jing, C. Y.; Jiang, G. B. ACS Appl. Mater. Inter. 2014, 6, 19833.
[45] Xiu, Z. M.; Ma, J.; Alvarez, P. J. J. Environ. Sci. Technol. 2011, 45, 9003.
[46] Xu, H. Y.; Qu, F.; Xu, H.; Lai, W. H.; Wang, Y. A.; Aguilar, Z. P.; Wei, H. Biometals 2012, 25, 45.
[47] He, D.; Dorantes-Aranda, J. J; Waite, T. D. Environ. Sci. Technol. 2012, 46, 8731.
[48] Bryaskova, R.; Pencheva, D.; Nikolov, S.; Kantardjiev, T. J. Chem. Biol. 2011, 4, 185.
[49] Masscheleyn, P. H.; Delaune, R. D.; Patrick, W. H. Environ. Sci. Technol. 1991, 25, 1414.
/
| 〈 |
|
〉 |