

Preparation and Characterization of Highly Stable and Aqueous Dispersion of Conjugated Polyelectrolyte/Single-Walled Carbon Nanotube Nanocomposites
Received date: 2018-03-06
Online published: 2018-04-20
Supported by
Project supported by the National Natural Science Foundation of China (Nos. 21674024, 21274029, 21320102005) and Ministry of Science and Technology of China (No. 2016YFA0203301).
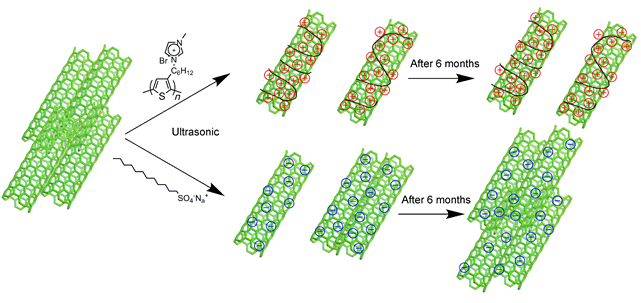
The dispersion of single-walled carbon nanotubes (SWNTs) is a key point to develop their extensive applications. Especially, to meet the requirements of future green chemistry, the preparation of environmentally-friendly, highly stable and well-distributed SWNTs in aqueous solution becomes particularly important. Based on it, a water-soluble conjugated polyelectrolyte, namely poly[3-[6-(N-methylimidazolium)hexyl]thiophene] (P3MHT) was designed and used to disperse SWNTs through non-covalent strategy. P3MHT was synthesized by a modified Grignard metathesis (GRIM) polymerization followed by quaternization of the bromohexyl side groups of the poly[3-(6-bromohexyl)thiophene] with N-methylimidazole. The P3MHT/SWNTs nanocomposites were prepared by mixing P3MHT and SWNTs in water during ultrasonication followed by centrifugation. UV-vis absorption spectroscopy, photoluminescence (PL) spectroscopy, transmission electron microscope (TEM), Zeta-nano electric potential analyzer, thermogravimetric (TGA) analysis were applied to characterize P3MHT/SWNTs nanocomposites. Compared to the commercial sodium dodecyl sulfate (SDS) surfactant to disperse SWNTs in aqueous solution, P3MHT exhibited a much better ability to disperse SWNTs under the same condition, i.e., the concentration of SWNTs dispersed by P3MHT was about two times than that of SWNTs dispersed by SDS. In P3MHT/SWNTs nanocomposite solution, SWNTs were exfoliated to form individuals or small bundles with an average size of 298 nm. However, in SDS/SWNTs solution, SWNTs preferred to form small aggregates with an average size of more than 500 nm. The P3MHT backbones were wrapped around individual SWNTs via π-π interactions to form the charge-transfer complexes. The ionic side chains of P3MHT not only made the nanocomposites dispersed in water, but also prevented the aggregation of SWNTs by electrostatic repulsion, resulting in aqueous dispersion of P3MHT/SWNTs nanocomposites. While SDS molecules were adsorbed on the surface of SWNTs via hydrophobic alkyl chains, which was much weaker than the π-π interactions between P3MHT and SWNTs. Such P3MHT/SWNTs nanocomposite solution exhibited high stability which remained almost unchanged after 6 months while SDS/SWNTs nanocomposite had already precipitated then. Overall, it provides a promising and simple method to develop highly stable and water processed SWNTs.
Zhu Mingjing , Peng Juan , Tang Ping , Qiu Feng . Preparation and Characterization of Highly Stable and Aqueous Dispersion of Conjugated Polyelectrolyte/Single-Walled Carbon Nanotube Nanocomposites[J]. Acta Chimica Sinica, 2018 , 76(6) : 453 -459 . DOI: 10.6023/A18030090
[1] Jariwala, D.; Sangwan, V. K.; Lauhon, L. J.; Marks, T. J.; Hersam, M. C. Chem. Soc. Rev. 2013, 42, 2824.
[2] Qiu, C.; Zhang, Z.; Xiao, M.; Yang, Y.; Zhong, D.; Peng, L. M. Science 2017, 355, 271.
[3] Liu, S.; Guo, X. F. Acta Chim. Sinica 2013, 71, 478(in Chinese). (刘松, 郭雪峰, 化学学报, 2013, 71, 478.)
[4] Zhang, S.; Kang, L.; Wang, X.; Tong, L.; Yang, L.; Wang, Z.; Qi, K.; Deng, S.; Li, Q.; Bai, X.; Ding, F.; Zhang, J. Nature 2017, 543, 234.
[5] Yang, F.; Wang, X.; Zhang, D.; Yang, J.; Luo, D.; Xu, Z.; Peng, F.; Li, X.; Li, R.; Li, Y.; Li, M.; Bai, X.; Ding, F.; Li, Y. Nature 2014, 510, 522.
[6] Li, L.; Jia, G. X.; Wang, X. X.; Wu, T. W.; Song, X. W.; An, S. L. Acta Chim. Sinica 2017, 75, 284(in Chinese). (李磊, 贾桂霄, 王晓霞, 吴铜伟, 宋希文, 安胜利, 化学学报, 2017, 75, 284.)
[7] R?sner, B.; Guldi, D. M.; Chen, J.; Minett, A. I.; Fink, R. H. Nanoscale 2014, 6, 3695.
[8] Sang, W. K.; Kim, T.; Kim, Y. S.; Hong, S. C.; Lim, H. J.; Yang, S. J.; Park, C. R. Carbon 2012, 50, 3.
[9] Guo, L. H.; Gong, L. H.; Yuan, F. L.; Zhang, B.; Bai, X. D.; Lian, Y. F. Acta Chim. Sinica 2005, 63, 1936(in Chinese). (郭丽华, 宫丽红, 袁福龙, 张斌, 白续铎, 廉永福, 化学学报, 2005, 63, 1936.)
[10] Lin, G. F.; Meng, L. J.; Zhang, X. K.; Lu, Q. H. Prog. Chem. 2010, 22, 331(in Chinese). (林高锋, 孟令杰, 张晓科, 路庆华, 化学进展, 2010, 22, 331.)
[11] Singh, P.; Campidelli, S.; Giordani, S.; Bonifazi, D.; Bianco, A.; Prato, M. Chem. Soc. Rev. 2009, 38, 2214.
[12] Liang, L.; Xie, W.; Fang, S.; He, F.; Yin, B.; Tlili, C.; Wang, D.; Qiu, S.; Li, Q. J. Mater. Chem. C 2017, 5, 11339.
[13] Samanta, S. K.; Fritsch, M.; Scherf, U.; Gomulya, W.; Bisri, S. Z.; Loi, M. A. Acc. Chem. Res. 2014, 47, 2446.
[14] Lee, H. W.; Yoon, Y.; Park, S.; Oh, J. H.; Hong, S.; Liyanage, L. S.; Wang, H.; Morishita, S.; Patil, N.; Park, Y. J.; Spakowitz, A.; Galli, G.; Gygi, F.; Wong, P. H.-S.; Tok, J. B.-H.; Kim, J. M.; Bao, Z. Nat. Commun. 2011, 2, 541.
[15] Yang, H.; Bezugly, V.; Kunstmann, J.; Filoramo, A.; Cuniberti, G. ACS Nano 2015, 9, 9012.
[16] Liu, Z.; Li, H.; Qiu, Z.; Zhang, S. L.; Zhang, Z. B. Adv. Mater. 2012, 24, 3633.
[17] Yang, S.; Meng, D.; Sun, J.; Yan, H.; Yong, H.; Geng, J. ACS Appl. Mater. Interfaces 2014, 6, 7686.
[18] Derenskyi, V.; Gomulya, W.; Rios, J. M.; Fritsch, M.; Fr?hlich, N.; Jung, S.; Allard, S.; Bisri, S. Z.; Gordiichuk, P.; Herrmann, A.; Scherf, U.; Loi, M. A. Adv. Mater. 2014, 26, 5969.
[19] Mai, C. K.; Liu, J.; Evans, C. M.; Segalman, R. A.; Chabinyc, M. L.; Cahill, D. G.; Bazan, G. C. Macromolecules 2016, 49, 4957.
[20] Li, Y.; Mai, C. K.; Phan, H.; Liu, X.; Nguyen, T. Q.; Bazan, G. C.; Chan-Park, M. B. Adv. Mater. 2014, 26, 4697.
[21] Kang, Y. K.; Lee, O. S.; Deria, P.; Sang, H. K.; Park, T. H.; Bonnell, D. A.; Saven, J. G.; Therien, M. J. Nano Lett. 2009, 9, 1414.
[22] Deria, P.; Olivier, J. H.; Park, J.; Therien, M. J. J. Am. Chem. Soc. 2014, 136, 14193.
[23] Mai, C. K.; Russ, B.; Fronk, S. L.; Hu, N.; Chan-Park, M. B.; Urban, J. J.; Segalman, R. A.; Chabinyc, M. L.; Bazan, G. C. Energy Environ. Sci. 2015, 8, 2341.
[24] Osaka, I.; Mccullough, R. D. Acc. Chem. Res. 2008, 41, 1202.
[25] He, M.; Zhao, L.; Wang, J.; Han, W.; Yang, Y.; Qiu, F.; Lin, Z. ACS Nano 2010, 4, 3241.
[26] He, M.; Ge, J.; Lin, Z.; Feng, X.; Wang, X.; Lu, H.; Yang, Y.; Qiu, F. Energy Environ. Sci. 2012, 5, 8351.
[27] Pan, S.; He, L.; Peng, J.; Qiu, F.; Lin, Z. Angew. Chem. Int. Ed. 2016, 55, 8686.
[28] Yang, X.; Ge, J.; He, M.; Ye, Z.; Liu, X.; Peng, J.; Qiu, F. Macromolecules 2016, 49, 287.
[29] Zhu, M.; Kim, H.; Yu, J. J.; Park, S.; Du, Y. R.; Kim, K.; Tang, P.; Qiu, F.; Kim, D. H.; Peng, L. J. Mater. Chem. A 2016, 4, 18432.
[30] Xia, H.; Ye, Z.; Liu, X.; Peng, J.; Qiu, F. RSC Adv.2014, 4, 19646.
[31] Duarte, A.; Pu, K. Y.; Liu, B.; Bazan, G. C. Chem. Mater. 2011, 23, 501.
[32] Feng, L.; Zhu, C.; Yuan, H.; Liu, L.; Lv, F.; Wang, S. Chem. Soc. Rev. 2013, 42, 6620.
[33] Rochat, S.; Swager, T. M. ACS Appl. Mater. Interfaces 2013, 5, 4488.
[34] Ghoos, T.; Malinkiewicz, O.; Conings, B.; Lutsen, L.; Vanderzande, D. J.; Bolink, H. J.; Maes, W. RSC Adv. 2013, 3, 25197.
[35] Liang, S.; Zhao, Y.; Adronov, A. J. Am. Chem. Soc. 2014, 136, 970.
[36] Bounioux, C.; Bar-Hen, A.; Yerushalmi-Rozen, R. Chem. Commun. 2015, 51, 6343.
[37] Chen, J.; Liu, H.; Weimer, W. A.; Halls, M. D.; Waldeck, D. H.; Walker, G. C. J. Am. Chem. Soc. 2002, 124, 9034.
[38] Rao, G. P.; Lu, C.; Su, F. Sep. Purif. Technol. 2007, 58, 224.
/
| 〈 |
|
〉 |