

Synthesis and Properties of an All-Conjugated Polythio-phene-Polyselenophene Diblock Copolymer
Received date: 2018-04-28
Online published: 2018-07-12
Supported by
Project supported by the National Natural Science Foundation of China (Nos. 21674024, 21320102005) and Ministry of Science and Technology of China (No. 2016YFA0203301).
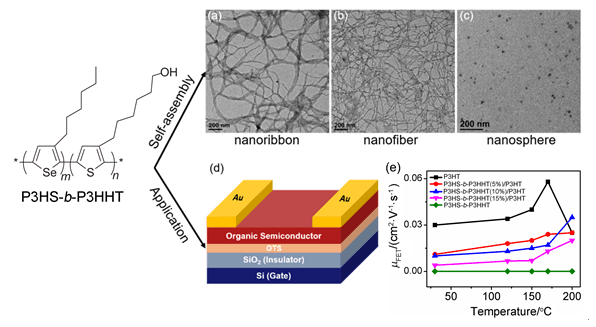
All-conjugated rod-rod block copolymers (BCPs) have gained immense interest over the past few years because they combine fascinating self-assembly properties of BCPs with the optical and electronic properties of conjugated polymers. Based on it, an all-conjugated rod-rod BCPs, poly(3-hexylselenophene)-b-poly[3-(6-hydroxyl)hexylthiophene] (P3HS-b-P3HHT) with hydroxyl groups as side substitution groups was synthesized via the Grignard metathesis (GRIM) method. The introduction of side hydroxyl groups was designed to endow different polarity between P3HS and P3HHT blocks and enrich the solution structures of P3HS-b-P3HHT. During thermal annealing, the cross-linking of hydroxyl groups was also utilized to improve the thermal stability of poly(3-hexylthiophene) (P3HT)-based organic field-effect transistors (OFETs) when blended with a certain amount of P3HS-b-P3HHT. On one hand, the use of mixed solvents provided an effective way to control the self-assembly behavior of P3HS-b-P3HHT. Depending on the mixed solvent ratio (i.e., chloroform/pyridine or methanol/pyridine), the rod-rod interaction of the copolymer chains was controlled, yielding a series of nanostructures such as nanoribbons, nanofibers, and nanospheres. Detailed morphologies and the corresponding photophysical behavior of different nanostructures were characterized by transmission electron microscope and UV-vis absorption spectra. The conformations of the P3HS and P3HHT chains in the solutions influenced their photophysical properties greatly. On the other hand, based on the thermal cross-linkable properties of hydroxyl groups, a certain amount of P3HS-b-P3HHT was mixed with P3HT homopolymer to fabricate P3HS-b-P3HHT/P3HT OFETs. For control samples, the charge carrier mobility of pure P3HT-based OFETs was improved with the increased annealed temperatures up to 170℃, then decreased significantly when the temperature further increased to 200℃. While overall, the charge carrier mobilities of P3HS-b-P3HHT/P3HT OFETs were lower than those of pure P3HT-based OFETs, they were improved with the increased temperature to 200℃. It was found the P3HS-b-P3HHT(10%)/P3HT OFETs exhibited the charge carrier mobility of 0.040 cm2·V-1·s-1 after annealing at 200℃ for 1 h, which was higher than P3HT OFETs (0.025 cm2·V-1·s-1) under the same experimental condition. It was due to the cross-linking of hydroxyl groups in P3HS-b-P3HHT retain the crystallization structures of P3HT, thus improved the thermal stability of OFETs. Overall, this work demonstrates a new polythiophene-polyselenophene BCP with controlled nanostructures by solvent blending and promising application in OFETs to improve their thermal stability.
Cui Huina , Qiu Feng , Peng Juan . Synthesis and Properties of an All-Conjugated Polythio-phene-Polyselenophene Diblock Copolymer[J]. Acta Chimica Sinica, 2018 , 76(9) : 691 -700 . DOI: 10.6023/A18040178
[1] Horowitz, G. Adv. Mater. 1998, 10, 365.
[2] Kim, N. K.; Jang, S. Y. G.; Caironi, P. M.; Park, W. T.; Khim, D.; Kim, J.; Kim, D. Y.; Noh, Y. Y. Chem. Mater. 2015, 27, 8345.
[3] Yang, N.; Qiao, X.; Fang, R.; Tao, J.; Hao, J.; Li, H. Acta Chim. Sinica 2016, 74, 335. (杨宁, 乔小兰, 房忍忍, 陶竞炜, 郝建, 李洪祥, 化学学报, 2016, 74, 335.)
[4] Lu, Y.; Ding, Y.; Wang, J. Chin. J. Org. Chem. 2016, 36, 2272. (卢阳, 丁一凡, 王婕妤, 裴坚, 有机化学, 2016, 36, 2272.)
[5] Günes, S.; Neugebauer, H.; Sariciftci, N. S. Chem. Rev. 2007, 107, 1324.
[6] Zhao, C.; Wang, Z.; Zhou, K.; Ge, H.; Zhang, Q.; Jin, L.; Wang, W.; Yin, S. Acta Chim. Sinica 2016, 74, 251. (赵蔡斌, 王占领, 周科, 葛红光, 张强, 靳玲侠, 王文亮, 尹世伟, 化学学报, 2016, 74, 251.)
[7] Nian, Y.; Wang, Z.; Jiang, H.; Feng, S.; Li, S.; Zhang, L.; Cao, Y.; Chen, Y. Chin. J. Chem. 2018, 36, 495.
[8] Perepichka, I. F.; Perepichka, D. F.; Meng, H.; Wudl, F. Adv. Mater. 2005, 17, 2281.
[9] Boudouris, B. W.; Frisbie, C. D.; Hillmyer, M. A. Macromolecules 2008, 41, 67.
[10] Wang, H.; Tang, G.; Jin, S.; Bian, C.; Han, F.; Liang, D.; Xue, X.; Acta Chim. Sinica 2007, 65, 2454. (王红敏, 唐国强, 晋圣松, 边成香, 韩菲菲, 梁丹, 徐学诚, 化学学报, 2007, 65, 2454.)
[11] Brinkmann, M.; Wittmann, J. C. Adv. Mater. 2006, 18, 860.
[12] Scherf, U.; Gutacker, A.; Koenen, N. Acc. Chem. Res. 2008, 41, 1086.
[13] Kim, J.; Song, I. Y.; Park, T. Chem. Commun. 2011, 47, 4697.
[14] Park, J. Y.; Koenen, N.; Forster, M.; Ponnapati, R.; Scherf, U.; Advincular, R. Macromolecules. 2008, 41, 6169.
[15] Thomas, A.; Houston, J. E.; Van der Brande N.; Winter, J. D.; Chevrer, M.; Heenan, R. K.; Terry, A. E.; Richeter, S.; Mehdi, A.; Mele, B. V.; Dubois, P.; Lazzaroni, R.; Gerbaux, P.; Evans, R. C.; Clement, S. Polym. Chem. 2014, 5, 3352.
[16] Li, Z.; Huo, Y.; Yang, X.; Ji, S. Chin. J. Org. Chem. 2016, 36, 2317. (李宗植, 霍延平, 阳香华, 籍少敏, 有机化学, 2016, 36, 2317.)
[17] Liu, Y.; Yuan, J.; Zou, Y.; Li, Y. Acta Chim. Sinica 2017, 75, 257. (刘晔, 袁俊, 邹应萍, 李永舫, 化学学报, 2017, 75, 257.)
[18] Shao, R.; Yang, X.; Yin, S.; Wang, W. Acta Chim. Sinica 2016, 74, 676. (邵绒, 杨鑫博, 尹世伟, 王文亮, 化学学报, 2016, 74, 676.)
[19] Li, H.; Fang, M.; Xu, T.; Hou, Y.; Tagn, R.; Chen, J.; Liu, L.; Han, H.; Peng, T.; Li, Q.; Li, Z. Org. Chem. Front. 2016, 2, 233.
[20] Gutacker, A.; Adamczyk, S.; Helfer, A.; Garner, L. E.; Evans, R. C.; Fonseca, S. M.; Knaapila, M.; Bazan, G. C.; Burrows, H. D.; Scherf. U. J. Mater. Chem. 2010, 20, 1423.
[21] Lai, Y. C.; Ohshimizu, K.; Takahashi, A.; Hsu, J. C.; Higashihara, T.; Ueda, M.; Chen, W. C. J. Polym. Sci., Part A:Polym. Chem. 2011, 49, 2577.
[22] Yu, X.; Xiao, K.; Chen, J.; Lavrik, N. V.; Hong, K.; Sumpter, B. G.; Geohegan, D. B. ACS Nano 2011, 5, 3559.
[23] Gilroy, J. B.; Lunn, D. J.; Patra, S. K.; Whittell, G. R.; Winnik, M. A.; Manners, I. Macromolecules 2012, 45, 5806.
[24] Moon, H. C.; Anthonysamy, A.; Kim, J. K. Macromolecules 2011, 44, 1894.
[25] Verduzco, R.; Botiz, I.; Pickel, D. L.; Kilbey, M. S. Hong, K.; Dimasi, E.; Darling, S. B. Macromolecules 2011, 44, 530.
[26] Ge, J.; He, M.; Xie, N.; Ye, Z.; Qiu, F. Macromolecules 2015, 48, 279.
[27] Wu, P. T.; Ren, G.; Li, C.; Mezzenga, R.; Jenekhe, S. A. Macromolecules 2009, 42, 2317.
[28] Yang, H.; Zhang, R.; Wang, L.; Zhang, J.; Yu, X.; Geng, Y.; Han, Y. Polymer 2016, 97, 238.
[29] Song, I. Y.; Kim, J.; Im, M. J.; Moon, B. J.; Park, T. Macromolecules 2012, 45, 5058.
[30] Scherf, U.; Adamczyk, S.; Gutacker, A.; Koenen, N. Macromol. Rapid Commun. 2009, 30, 1059.
[31] Ho, V.; Boudouris, B. W.; Segalman, R. A. Macromolecules 2010, 43, 7895.
[32] He, M.; Han, W.; Ge, J.; Yang, X.; Qiu, F.; Lin, Z. Energy Environ. Sci. 2011, 4, 2894.
[33] Yang, X.; Ge, J.; He, M.; Ye, Z.; Peng, J.; Qiu, F. Macromolecules 2016, 49, 287.
[34] Zhu, M.; Kim, H.; Jang, Y. J.; Park, S.; Ryu, D. Y.; Kim, K.; Tang, P.; Qiu, F.; Kim, D. H.; Peng, J. J. Mater. Chem. A 2016, 4, 18432.
[35] Xia, H.; Ye, Z.; Liu, X.; Peng, J.; Qiu, F. RSC Adv. 2014, 4, 19646.
[36] Wang, Y.; Cui, H.; Zhu, M.; Peng, J.; Lin, Z. Macromolecules 2017, 50, 9674.
[37] Hollinger, J.; Jahnke, A. A.; Coombs, N.; Seferos, D. S. J. Am. Chem. Soc. 2010, 132, 8546.
[38] Patra, A.; Bendikov, M. J. Mater. Chem. 2010, 20, 422.
[39] Ge, J.; He, M.; Yang, X.; Qiu, F. Macromolecules 2010, 43, 6422.
[40] Yang, H.; Xia, H.; Wang, G.; Peng, J. J. Polym. Sci., Part A:Polym. Chem. 2012, 50, 5060.
[41] He, L.; Pan, S.; Peng, J. J. Polym. Sci., Part B:Polym. Phys. 2016, 54, 544.
[42] Li, L.; Hollinger, J.; Jahnke, A.; Petrov, S.; Seferos, S. Chem. Sci. 2011, 2, 2306.
[43] Liu, J.; Arif, M.; Zou, J.; Khondaker, S.; Zhai, L. Macromolecules 2009, 42, 9390.
/
| 〈 |
|
〉 |