

Enrichment of U(VI) on Polyaniline Modified Mxene Composites Studied by Batch Experiment and Mechanism Investigation
Received date: 2018-06-24
Online published: 2018-07-27
Supported by
Project supported by the financial support from the National Key Research and Development Program of China (No. 2017YFA0207002), National Natural Science Foundation of China (Nos. 21577032 and 21707033) and the NCEPU "Double First-Class" Graduate Talent Cultivation Program (No. 035/XM1805316).
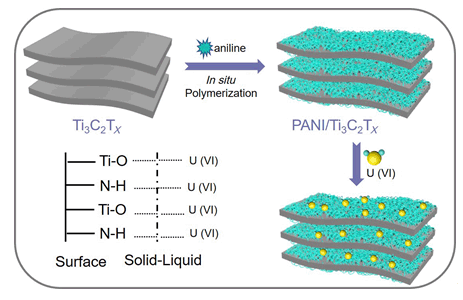
Remediation of nuclear wastewater containing U(VI) is very important to human health and environmental ecosystems. Recently, numerous kinds of adsorbents such as clay minerals, carbon-based material and layered double hydroxides etc. have been extensively investigated for effective containing U(VI) wastewater treatment. A representative class of two-dimensional material, "Mxene" has received multidisciplinary interests due to their widespread application in the fields of batteries, supercapacitors and wastewater treatment. Unfortunately, the adsorption capacity of pristine Mxene is frequently limited due to the low quantity of surface functional groups. It was obviously that synthesizing functionalized Mxene materials with plenty functional groups is of great importance for wastewater remediation. In this manuscript, polyaniline modified Mxene composites (PANI/Ti3C2Tx) were successfully synthesized by a in situ polymerization method and were characterized by a series of methods including SEM, FT-IR, XRD and XPS techniques. The adsorption behavior of U(VI) on PANI/Ti3C2Tx was systematically explored by batch experiment. The experiment results showed that the removal process was obviously affected by the ion strength, indicating the formation of outer-sphere surface complexes. Meanwhile, the thermodynamic results manifested that the adsorption process was spontaneous and endothermic reaction. Based on Langmuir model fit, the maximum adsorption capacity of U(VI) on polyaniline modified Mxene composites was calculated to be 102.8 mg/g at pH=5.0 and 298 K, which was superior than that of U(VI) on pristine Ti3C2Tx (36.6 mg/g). In addition, spectroscopy characterizations including Fourier transform infrared spectrometry and X-ray photoelectron spectroscopy were applied to study the underlying interaction mechanism, which was mainly attributed to the strong surface complexion between surface functional groups (oxygen-containing groups and amino groups) and U(VI). This work herein pointed out that PANI/Ti3C2Tx materials were promising adsorbent for the efficient removal of U(VI) in the environmental pollution remediation.
Key words: PANI/Ti3C2Tx; U(VI); adsorption; interaction mechanism
Gu Pengcheng , Song Shuang , Zhang Sai , Wei Benben , Wen Tao , Wang Xiangke . Enrichment of U(VI) on Polyaniline Modified Mxene Composites Studied by Batch Experiment and Mechanism Investigation[J]. Acta Chimica Sinica, 2018 , 76(9) : 701 -708 . DOI: 10.6023/A18060245
[1] Ma, L.; Wang, Q.; Islam, S.; Liu, Y.; Ma, S.; Kanatzidis, M. J. Am. Chem. Soc. 2016, 138, 2858.
[2] Manos, M.; Kanatzidis, M. J. Am. Chem. Soc. 2012, 134, 16441.
[3] Song, S.; Huang, S.; Zhang, R.; Chen, Z.; Wen, T.; Wang, S.; Alsaedi, A.; Hayat, T.; Wang, X. Chem. Eng. J. 2017, 325, 576.
[4] Pang, H.; Wang, X.; Yao, W.; Yu, S.; Wang, X. Sci. China. Chem. 2018, 48, 58. (庞宏伟, 王祥学, 姚文, 于淑君, 王祥科, 中国科学, 化学, 2018, 48, 58.)
[5] Chen, H.; Chen, Z.; Zhao, G.; Zhang, Z.; Xu, C.; Liu, Y.; Chen, J.; Zhuang, L.; Haya, T.; Wang, X. J. Hazard. Mater. 2018, 347, 67.
[6] Chen, H.; Huang, S.; Zhang, Z.; Liu, Y.; Wang, X. Acta Chim. Sinica 2017, 75, 560. (陈海军, 黄舒怡, 张志宾, 刘云海, 王祥科, 化学学报, 2017, 75, 560.)
[7] Favre-Réguillon, A.; Lebuzit, G.; Murat, D.; Foos, J.; Mansour, C.; Draye, M. Water. Res. 2008, 42, 1160.
[8] Gu, P.; Zhang, S.; Li, X.; Wang, X.; Wen, T.; Jehan, R.; Alsaedi, A.; Wang, X. Environ. Pollut. 2018, 240, 493.
[9] Li, J.; Gong, L.; Feng, X.; Zhang, L.; Wu, H.; Yan, C.; Xiong, Y.; Gao, H.; Luo, F. Chem. Eng. J. 2017, 316, 154.
[10] Gu, P.; Xing, J.; Wen, T.; Zhang, R.; Wang, J.; Zhao, G.; Hayat, T.; Ai, Y.; Lin, Z.; Wang, X. Environ. Sci. Nano 2018, 5, 946.
[11] Liang, Y.; Gu, P.; Yao, W.; Yu, S.; Wang, J.; Wang, X. Prog. Chem. 2017, 29, 1062. (梁宇, 顾鹏程, 姚文, 于淑君, 王建, 王祥科, 化学进展, 2017, 29, 1062.)
[12] Zhao, G.; Li, J.; Ren, X.; Chen, C.; Wang, X. Environ. Sci. Technol. 2011, 45, 10454.
[13] Sun, Y.; Shao, D.; Chen, C.; Yang, S.; Wang, X. Environ. Sci. Technol. 2013, 47, 9904.
[14] Mashtalir, O.; Naguib, M.; Mochalin, V.; Dall'Agnese, Y.; Heon, M.; Barsoum, M.; Gogotsi, Y. Nat. Commun. 2013, 4, 1716.
[15] Naguib, M.; Gogotsi, Y. Acc. Chem. Res. 2014, 48, 128.
[16] Naguib, M.; Come, J.; Dyatkin, B.; Presser, V.; Taberna, P.; Si-mon, P.; Barsoum, M.; Gogotsi, Y. Electrochem. Commun. 2012, 16, 61.
[17] Lukatskaya, M.; Mashtalir, O.; Ren, C.; Dall'Agnese, Y.; Rozier, P.; Taberna, P.; Naguib, M.; Simon, P.; Barsoum, M.; Gogotsi, Y. Science 2013, 341, 1502.
[18] Ma, T.; Cao, J.; Jaroniec, M.; Qiao, S. Angew. Chem. Int. Ed. 2016, 55, 1138.
[19] Ying, Y.; Liu, Y.; Wang, X.; Mao, Y.; Cao, W.; Hu, P.; Peng, X. ACS Appl. Mater. Inter. 2015, 7, 1795.
[20] Fard, A.; McKay, G.; Chamoun, R.; Rhadfi, T.; Preud'Homme, H.; Atieh, M. Chem. Eng. J. 2017, 317, 331.
[21] Wang, L.; Yuan, L.; Chen, K.; Zhang, Y.; Deng, Q.; Du, S.; Huang, Q.; Zheng, R.; Zhang, J.; Chai, Z.; Barsoum, M.; Wang, X.; Shi, W. ACS Appl. Mater. Inter. 2016, 8, 16396.
[22] Wang, L.; Tao, W.; Yuan, L.; Liu, Z.; Huang, Q.; Chai, Z.; Gibson, J.; Shi, W. Chem. Commun. 2017, 53, 12084.
[23] Peng, Q.; Guo, J.; Zhang, Q.; Xiang, J.; Liu, B.; Zhou, A.; Liu, R.; Tian, Y. J. Am. Chem. Soc. 2014, 136, 4113.
[24] Naguib, M.; Kurtoglu, M.; Presser, V.; Lu, J.; Niu, J.; Heon, M.; Hultman, L.; Gogotsi, Y.; Barsoum, M. Adv. Mater. 2011, 23, 4248.
[25] Yu, S.; Wang, X.; Chen, Z.; Wang, J.; Wang, S.; Hayat, T.; Wang, X. J. Hazard. Mater. 2017, 321, 111.
[26] Pang, H.; Huang, S.; Wu, Y.; Yang, D.; Wang, X.; Yu, S.; Chen, Z.; Alsaedi, A.; Hayat, T.; Wang, X. Inorg. Chem. Front. 2018 DOI:10. 1039/C8QI00253C.
[27] Zhu, K.; Lu, S.; Gao, Y.; Zhang, R.; Tan, X.; Chen, C. Appl. Surf. Sci. 2017, 396, 1726.
[28] Yao, W.; Wang, X.; Liang, Y.; Yu, S.; Gu, P.; Sun, Y.; Xu, C.; Chen, J.; Hayat, T.; Alsaedi, A.; Wang, X. Chem. Eng. J. 2018, 332, 775.
[29] Song, S.; Yin, L.; Wang, X.; Liu, L.; Huang, S.; Zhang, R.; Wen, T.; Yu, S.; Fu, D.; Hayat, T.; Wang, X. Chem. Eng. J. 2018, 338, 579.
[30] Yang, S.; Wang, X.; Chen, Z.; Li, Q.; Wei, B.; Wang, X. Prog. Chem. 2018, 30, 225. (杨姗也, 王祥学, 陈中山, 李倩, 韦犇犇, 王祥科, 化学进展, 2018, 30, 225.)
[31] Wen, T.; Wu, X.; Tan, X.; Wang, X.; Xu, A. ACS Appl. Mater. Inter. 2013, 5, 3304.
[32] Yang, D.; Wang, X.; Wang, N.; Zhao, G.; Song, G.; Chen, D.; Liang, Y.; Wen, T.; Wang, H.; Hayat, T.; Alsaedi, A.; Wang, X.; Wang, S. J. Clean. Prod. 2017, 172, 2033.
[33] Yu, S.; Wang, J.; Song, S.; Sun, K.; Li, J.; Wang, X.; Chen, Z.; Wang, X. Sci. China. Chem. 2017, 60, 415.
[34] Ma, S.; Huang, L.; Ma, L.; Shim, Y.; Islam, S.; Wang, P.; Zhao, L.; Wang, S.; Sun, G.; Yang, X.; Kanatzidis, M. J. Am. Chem. Soc. 2015, 137, 3670.
[35] Zou, Y.; Wang, P.; Yao, W.; Wang, X.; Liu, Y.; Yang, D.; Wang, L.; Hou, J.; Alsaedi, A.; Hayat, T.; Wang, X. Chem. Eng. J. 2017, 330, 573.
[36] Zhang, C.; Liu, Y.; Li, X.; Chen, H.; Wen, T.; Jiang, Z.; Ai, Y.; Sun, Y.; Hayat, T.; Wang, X. Chem. Eng. J. 2018, 346, 406.
[37] Zhou, T.; Li, C.; Jin, H.; Lian, Y.; Han, W. ACS Appl. Mater. Inter. 2017, 9, 6030.
[38] Zou, Y.; Wang, X.; Wu, F.; Yu, S.; Hu, Y.; Song, W.; Liu, Y.; Wang, H.; Hayat, T.; Wang, X. ACS Sustain. Chem. Eng. 2016, 5, 1173.
[39] Han, M.; Yin, X.; Wu, H.; Hou, Z.; Song, C.; Li, X.; Zhang, L.; Cheng, L. ACS Appl. Mater. Inter. 2016, 8, 21011.
[40] Shao, D.; Hou, G.; Li, J.; Wen, T.; Ren, X.; Wang, X. Chem. Eng. J. 2014, 255, 604.
[41] Franczyk, T.; Czerwinski, K.; Raymond, K. J. Am. Chem. Soc. 1992, 114, 8138.
/
| 〈 |
|
〉 |