

Reductive Amination by One Pot Reaction of Aldehydes and Alkoxyamines Catalyzed by B(C6F5)3
Received date: 2018-07-18
Online published: 2018-08-13
Supported by
Project supported by the National Natural Science Foundation of China (21542011), and Scientific Research Fund of Leshan Normal University (Z1414, Z1308).
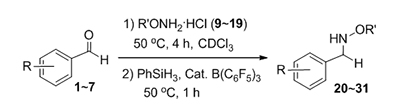
Recently the research work concerning B(C6F5)3 catalyzed reductive and amination of aldehydes and ketones revealed that this extremely electron-deficient borane is, actually, a rather water-tolerant catalyst. This fact considerably broadens the scope of the water/base tolerant FLP chemistry. In this project, an efficient one pot reductive amination method has been developed by reaction of aldehydes and alkoxyamines with hydrosilanes as the hydride sources and B(C6F5)3 as catalyst without cleavage of the N-O bond. This protocol can be used to prepare the secondary and tertiary alkoxyamines by starting from the primary and secondary ones, respectively. A special attention has been paid to elucidate the role of water in the reductive amination. When benzaldehyde reacts with benzoxylamine, only the condensation product oxime ether could be observed. Whereas surprisingly when excess amount of water is added, the reductive amination goes successfully like the alkoxyamine hydrochloride works. The detailed NMR data has shown that a transformation of the intermediate oximes ArCH=NOR to the "ammonium borates"[ArCH=NHOR]+[X-B(C6F5)3]-(X=Cl, OH) can take place in the reaction system, while the latter can be converted into the well-known active intermediate "ammonium hydroborates"[ArCH=NHOR]+[H-B(C6F5)3]- to reduce the C=N bond under mild condition in the presence of hydrosilanes. That means the deprotonation reaction of the Lewis acid water adduct H2O-B(C6F5)3 could be a key step for the B(C6F5)3 catalyzed reaction under moist condition. In this case the adduct H2O-B(C6F5)3 acts as a Brønsted acid as HCl does. Meanwhile a simulative experiment under different ratio of water has been fulfilled to prove this speculation. The C=N bond of Benzalaniline (PhCH=NPh) and Benzyloxy oxime ether (PhCH=NOCH2Ph) could be reduced only in presence of 2 equiv. H2O rather than equivalent. Based on this study it has shown that in the frustrated Lewis pair (FLP) chemistry, the Lewis acid B(C6F5)3 is not only a highly effective and water tolerant catalyst, the "disfavored" deprotonation of H2O-B(C6F5)3 adductis possibly playing an important role in reductive amination reaction. To clarify in detail the actual role of water in the reductive amination reaction under the "moist" conditions would enable the further development of FLP and related catalyzed reactions.
Key words: frustrated Lewis pairs; B(C6F5)3; hydrosilanes; reductive amination; water
He Yunqing , Teng Jinwei , Tian Chong , Borzov Maxim , Hu Qishan , Nie Wanli . Reductive Amination by One Pot Reaction of Aldehydes and Alkoxyamines Catalyzed by B(C6F5)3[J]. Acta Chimica Sinica, 2018 , 76(10) : 774 -778 . DOI: 10.6023/A18070281
[1] Parks, D. J.; Piers, W. E. J. Am. Chem. Soc. 1996, 118, 9440.
[2] Stephan, D. W.; Erker, G. Angew. Chem., Int. Ed. 2010, 49, 46.
[3] Liu, Y.-B.; Du, H.-F. Acta Chim. Sinica 2014, 72, 771. (刘勇兵, 杜海峰, 化学学报, 2014, 72, 771.)
[4] Oestreich, M.; Hermeke, J.; Mohr, J. Chem. Soc. Rev. 2015, 44, 2202.
[5] Stephan, D. W.; Erker, G. Angew. Chem., Int. Ed. 2015, 54, 6400.
[6] Scott, D. J.; Fuchter, M. J.; Ashley, A. E. J. Am. Chem. Soc. 2014, 136, 15813.
[7] Mahdi, T.; Stephan, D. W. J. Am. Chem. Soc. 2014, 136, 15809.
[8] Gyömöre, A.; Bakos, M.; Földes, T.; Papai, I.; Domja, N. A.; Soós, T. ACS Catal. 2015, 5, 5366.
[9] Fasano, V.; Radcliffe J. E.; Ingleson, M. J. ACS Catal. 2016, 6(3), 1793.
[10] Fasano, V.; Ingleson, M. J. Chem. Eur. J. 2017, 23(9), 2217.
[11] Melman, A. In The Chemistry of Hydroxylamines, Oximes and Hydroxamic Acids, Part 1, Eds.:Raqppoport, Z.; Liebman, J. F., Wiley, Chichester, 2009, pp. 117~161.
[12] Mohr, J.; Oestreich, M. Angew. Chem., Int. Ed. 2014, 53, 1.
[13] Mohr, J.; Porwal, D.; Chatterjee, I.; Oestreich, M. Chem. Eur. J. 2015, 21, 17583.
[14] Hu, X.; Tian, C.; Jiang, Y.; Borzov, M.; Nie, W.-L. Acta Chim. Sinica 2015, 73, 1025. (胡茜, 田冲, Borzov Maxim, 聂万丽, 化学学报, 2015, 73, 1025.)
[15] Tian, C.; Jiang, Y.; Borzov, M.; Nie, W.-L. Acta Chim. Sinica 2015, 73, 1203. (田冲, 姜亚, Borzov Maxim, 聂万丽, 化学学报, 2015, 73, 1203).
[16] Wen, Z.-G.; Tian, C.; Jiang, Y.; Borzov, M.; Nie, W.-L. Acta Chim. Sinica 2016, 74, 498. (温志国, 田冲, Borzov Maxim, 聂万丽, 化学学报, 2016, 74, 498).
[17] Nie, W.-L.; Sun, G.-F.; Tian, C.; Borzov, M. V. Z. Naturforsch. 2016, 71, 1029.
[18] Zhang, L.-W.; Wen, Z.-G.; Borzov, M.; Nie, W.-L. Acta Chim. Sinica 2017, 75, 819. (张露文, 温志国, Borzov Maxim, 聂万丽, 化学学报, 2017, 75, 819).
[19] Sun, G.-F.; Su, M.; Fang, J.; Borzov, M.; Nie, W.-L. Acta Chim. Sinica 2017, 75, 824. (孙国峰, 苏敏, 方洁, Borzov Maxim, 聂万丽, 化学学报, 2017, 75, 824).
[20] He, Y.-Q.; Zou, M.-Y.; Xue, Y.; Hu, Q.-S.; Borzov, M. V.; Nie, W.-L. Mechanism Aspects of the B(C6F5)3 Catalyzed Reductive Amination, 2018, Submitted.
[21] Beck, A. D. J. Chem. Phys. 1993, 98, 5648.
[22] Parr, R. G.; Yang, W. Density Functional Theory of Atoms and Molecules, Oxford University Press, Oxford, 1989.
[23] Reed, A. E.; Weinstock, R. B.; Weinhold, F. J. Chem. Phys. 1985, 83, 735.
[24] Reed, A. E.; Curtiss, L. A.; Weinhold, F. Chem. Rev. 1988, 88, 899.
[25] Frisch, M. J.; Trucks, G. W.; Schlegel, H. B.; Scuseria, G. E.; Robb, M. A.; Cheeseman, J. R.; Scalmani, G.; Barone, V.; Petersson, G. A.; Nakatsuji, H.; Li, X.; Caricato, M.; Marenich, A. V.; Bloino, J.; Janesko, B. G.; Gomperts, R.; Mennucci, B.; Hratchian, H. P.; Ortiz, J. V.; Izmaylov, A. F.; Sonnenberg, J. L.; Williams-Young, D.; Ding, F.; Lipparini, F.; Egidi, F.; Goings, J.; Peng, B.; Petrone, A.; Henderson, T.; Ranasinghe, D.; Zakrzewski, V. G.; Gao, J.; Rega, N.; Zheng, G.; Liang, W.; Hada, M.; Ehara, M.; Toyota, K.; Fukuda, R.; Hasegawa, J.; Ishida, M.; Nakajima, T.; Honda, Y.; Kitao, O.; Nakai, H.; Vreven, T.; Throssell, K.; Montgomery, J. A.; Jr., Peralta, J. E.; Ogliaro, F.; Bearpark, M. J.; Heyd, J. J.; Brothers, E. N.; Kudin, K. N.; Staroverov, V. N.; Keith, T. A.; Kobayashi, R.; Normand, J.; Raghavachari, K.; Rendell, A. P.; Burant, J. C.; Iyengar, S. S.; Tomasi, J.; Cossi, M.; Millam, J. M.; Klene, M.; Adamo, C.; Cammi, R.; Ochterski, J. W.; Martin, R. L.; Morokuma, K.; Farkas, O.; Foresman, J. B.; Fox, D. J. Gaussian 16, Revision A.03, Gaussian, Inc., Wallingford CT, 2016.
[26] Miertus, S.; Tomasi, J. Chem. Phys. 1982, 65, 239.
[27] Cossi, M.; Barone, V.; Cammi, R.; Tomasi, J. Chem. Phys. Lett. 1996, 255, 327.
[28] Nie, H.-F.; Zhong, H.-Y.; Li, X.-N.; Li, Y.-Q.; Wang, J.-X. Chin. J. Org. Chem. 2013, 33, 2412. (聂红芬, 周洪勇, 李小娜, 李云庆, 王家喜, 有机化学, 2013, 33, 2412.)
/
| 〈 |
|
〉 |