

Effect of Nitrogen-Containing Functional Groups of Cobalt Phthalocyanine Catalyst on the Oxygen Reduction Performance in Fuel Cells
Received date: 2018-06-12
Online published: 2018-08-14
Supported by
Project supported by the Special Foundation for Basic Scientific Research Business of East China University of Science and Technology (No. 222201814008), and the Shanghai Pujiang Talent Plan (No. 18PJ1402000).
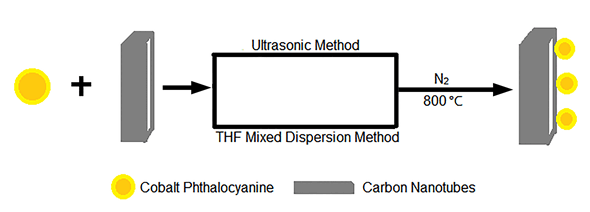
An ultrasonic method and a tetrahydrofuran-mixed dispersion method were used to synthesize two heat-treated cobalt phthalocyanine catalysts supported on carbon nanotubes, CoPc-CNT-S and CoPc-CNT-R,respectively. The ultrasonic process was that mixing cobalt phthalocyanine and carbon nanotubes in isopropanol under ultrasound condition within 30 min, while the tetrahydrofuran-mixed dispersion method was that mixing cobalt phthalocyanine and carbon nanotubes in tetrahydrofuran at 80℃ lasting 4 h. Then the pyrolysis process was carried out in a tube furnace under Argon (Ar) atmosphere with a heating rate of 5℃/min to 800℃ and lasting 2 h. Thermogravimetric Analysis (TGA) results showed that cobalt content of CoPc-CNT-S was 8.1 wt% while CoPc-CNT-R was 7.0 wt%. Moreover, X-ray photoelectron spectroscopy (XPS) results gave a conclusion that nitrogen content of CoPc-CNT-R (5.22%) is twice more than CoPc-CNT-S (2.08%). In comparsion with CoPc-CNT-R, CoPc-CNT-S has more pyrrole nitrogen on the surface. The fuel cell tests in a PEM/AEM hybrid fuel cell showed that the activity and stability of CoPc-CNT-S performed better than CoPc-CNT-R. Power density of CoPc-CNT-S hold at 18.6 mW/cm2 in H2/O2 hybrid AEM/PEM fuel cell for 15 h and CoPc-CNT-R can only hold at 9 mW/cm2. The current density of CoPc-CNT-S maintain at 68 mA/cm2 after stability test in H2/O2 hybrid AEM/PEM fuel cell for 20 h under 50 mV, but the stablity of CoPc-CNT-S fluctuate between 20 mA/cm2 to 40 mA/cm2. The reason can be concluded that ultrasonic method and tetrahydrofuran-mixed dispersion method can cause different kind of nitrogen doped on catalyst to influence electrocatalytic properties. The phenomenon that the electron transfer resistance of CoPc-CNT-S was lower than CoPc-CNT-R after working in PEM/AEM fuel cells for 5 h and 15 h can prove indirectly that the activity of CoPc-CNT-R using for the cathode catalyst H2/O2 hybrid AEM/PEM fuel cell is obviously less than CoPc-CNT-S. These observations may result from the cooperative effect from the similar ratio of pyridinic and pyrrolic nitrogen which may accelerate the catalytic activity of CoPc-CNT-S toward oxygen reduction reaction.
Huang Wenjiao , Zhang Haoyu , Hu Shuozhen , Niu Dongfang , Zhang Xinsheng . Effect of Nitrogen-Containing Functional Groups of Cobalt Phthalocyanine Catalyst on the Oxygen Reduction Performance in Fuel Cells[J]. Acta Chimica Sinica, 2018 , 76(9) : 723 -728 . DOI: 10.6023/A18060231
[1] Chang, Z.; Meng, F.; Zhong, H. Chin. J. Chem. 2018, 36, 287.
[2] Wilkinson, D. P.; Zhang, J.; Hui, R.; Fergus, J.; Li, X. Platinum Met. Rev. 2011, 55, 225.
[3] Zhao, T. S.; Li, Y. S.; Shen, S. Y. Front. Energy Power Eng. China. 2010, 4, 443.
[4] Merle, G.; Wessling, M.; Nijmeijer, K. J. Membr. Sci. 2011, 377, 1.
[5] Ciureanu, M.; Roberge, R. J. Phys. Chem. B 2001, 105, 3531.
[6] Steele, B. C.; Heinzel, A. Nature 2001, 414, 345
[7] Zhou, Y.; Neyerlin, K.; Olson, T. S.; Pylypenko, S.; Bult, J.; Dinh, H. N.; Gennett, T.; Hayre, R. O. Energy Environ. Sci. 2010, 10, 1437.
[8] Hayre, R. O. J. Mater. Chem. 2009, 19, 7830.
[9] Wu, J.; Yang, H. Acc. Chem. Res. 2013, 46, 1848.
[10] He, X.; Gang, M.; He, G. Chin. J. Chem. 2017, 35, 673.
[11] Nie, Y.; Li, L.; Wei, Z. Chem. Soc. Rev. 2015, 44, 2168.
[12] Wang, B. J. Power Sources 2005, 152, 1.
[13] Liu, J.; Li, E.; Ruan, M.; Song, P.; Xu, W. Catalysts 2015, 5, 1167.
[14] Titov, A.; Zapol, P.; Kral, P.; Liu, D. J.; Iddir, H.; Baishya, K.; Curtiss, L. A. J. Phys. Chem. C 2009, 113, 21629.
[15] Kruusenberg, I.; Mondal, J.; Matisen, L.; Sammelselg, V.; Tammeveski, K. Electrochem. Commun. 2013, 33, 18.
[16] Media, A.; Jang, J.; Kim, S.; Kim, J.; Peck, D.; Jung, D. Korean Chem. Soc. 2015, 36, 919.
[17] Huang, W.; Ahlfield, J. M.; Zhang, X.; Kohl, P. A. J. Electrochem. Soc. 2017, 164, 217.
[18] Zhang, R.; Peng, Y.; Li, Z.; Li, K.; Ma, J.; Liao, Y.; Zheng, L.; Zuo, X.; Xia, D. Electrochim. Acta 2014, 147, 343.
[19] Ania, C. O.; Seredych, M.; Rodriguez-Castellon, E.; Bandosz, T. J. Appl. Catal. B Environ. 2015, 163, 424.
[20] Huang, W.; Ahlfield, J. M.; Kohl, P. A.; Zhang, X. Electrochim. Acta 2017, 257, 224.
[21] Xu, X.; Peng, S.; Zhang, J.; Lu, S.; Xiang, Y. Acta Chim. Sinica 2016, 74, 271(in Chinese). (徐鑫, 彭思侃, 张劲, 卢善富, 相艳, 化学学报, 2016, 74, 271.)
[22] Ding, L.; Qiao, J.; Dai, X.; Zhang, J.; Zhang, J.; Tian, B. Int. J. Hydrogen Energy 2012, 37, 14103.
[23] Kruusenberg, I.; Matisen, L.; Shah, Q.; Kannan, A. M.; Tam-meveski, K. Int. J. Hydrogen Energy 2012, 37, 4406.
[24] Chen, R.; Li, H.; Chu, D.; Wang, G. J. Phys. Chem. C 2009, 113, 20689.
[25] Ünlü, M.; Zhou, J.; Kohl, P. A. Fuel Cells 2010, 10, 54.
[26] Ünlü, M. J. Phys. Chem. C 2009, 113, 11416.
[27] Yuan, P.; Chen, J.; Pan, D.; Bao, X. Acta Chim. Sinica 2016, 74, 603(in Chinese). (袁珮, 陈建, 潘登, 鲍晓军, 化学学报, 2016, 74, 603.)
[28] Shao, Y.; Zhang, S.; Engelhard, M. H.; Li, G.; Shao, G.; Wang, Y.; Liu, J.; Aksay, I. A.; Lin, Y. J. Mater. Chem. 2010, 20, 7491.
[29] Xing, T.; Zheng, Y.; Li, L. H.; Cowie, B. C. C.; Gunzelmann, D.; Qiao, S. Z.; Huang, S.; Chen, Y. ACS Nano 2014, 8, 6856.
[30] Artyushkova, K.; Serov, A.; Rojas-Carbonell, S.; Atanassov, P. J. Phys. Chem. C 2015, 119, 25917.
[31] Liang, W.; Chen, J.; Liu, Y.; Chen, S. ACS Catal. 2014, 4, 4170.
[32] Zhou, J.; Joseph, K.; Ahlfield, J. M.; Park, D. Y.; Kohl, P. A. J. Electrochem. Soc. 2013, 160, 573.
/
| 〈 |
|
〉 |