

Computational Study of the Trifluoromethyl Radical Donor Abilities of CF3 Sources
Received date: 2018-08-14
Online published: 2018-10-08
Supported by
Project supported by the National Natural Science Foundation of China (Nos. 21772098, 21390400), Collaborative Innovation Center of Chemical Science and Engineering (Tianjin), the State Key Laboratory on Elemento-Organic Chemistry, and the Fundamental Research Funds for the Central Universities.
Organic compounds containing trifluoromethyl (CF3) group(s) are widely prevalent in biochemical and medicinal science. This is mainly due to the fact that the trifluoromethyl group often improves the metabolic stability and lipophilicity of biologically active compounds. The need of efficient methods for the incorporation of this group into target molecules has spurred research to discover new, practical CF3 sources. Among various CF3 sources, the radical trifluoromethylating reagents has provided a strong driving force for the discovery of the novel trifluoromethylation reactions, and contributed enormously to the efficient synthesis of various CF3-containing compounds. Although a wide variety of radical CF3 sources are now available to organic chemists, little attention has been paid to assess their trifluoromethyl radical donor abilities (TR·DA). Moreover, the available radical reagents show a very rich and diverse reactivity. The establishment of an extensive scale to quantify their CF3 radical donating abilities should be of great value for both the rational design of novel reagents and the judicious selection of appropriate reagent to explore new radical reactions. Herein, we present a systematic computational study of the homolytic X―CF3 bond dissociation enthalpies of 35 radical trifluoromethylating reagents by using the SMD-M06-2X/[6-311++G(2df, 2p)-Def2-QZVPPD]//SMD-M06-2X/[6-31+G(d)-LANL2DZ] method, aiming to provide an energetic guide for estimating their trifluoromethyl radical donor abilities. A comprehensive TR·DA scale was constructed, which covers a range from -21.5 to 95.2 kcal·mol-1. The effects of the frequently used activators including single electron transfer reagents and halogen/chalcogen-bond donors on trifluoromethyl radical donor abilities were investigated. The results show that single electron transfer is the most efficient way to promote the CF3 radical release. We expect that the results of this study could be highly valuable for the mechanistic understanding and the rational design of novel CF3 sources and new radical trifluoromethylation reactions.
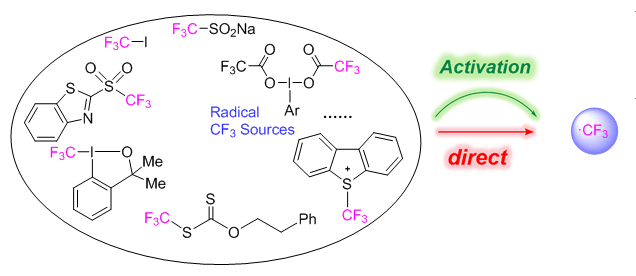
Li Man , Cheng Jin-Pei , Kang Huiying , Xue Xiao-Song , Cheng Jin-Pei . Computational Study of the Trifluoromethyl Radical Donor Abilities of CF3 Sources[J]. Acta Chimica Sinica, 2018 , 76(12) : 988 -996 . DOI: 10.6023/A18080334
[1] (a) Leo, A.; Hansch, C.; Elkins, D. Chem. Rev. 1971, 71, 525.
(b) Hansch, C.; Leo, A.; Unger, S. H.; Kim, K. H.; Nikaitani, D.; Lien, E. J. J. Med. Chem. 1973, 16, 1207.
(c) Hansch, C.; Leo, A.; Taft, R. W. Chem. Rev. 1991, 91, 165.
[2] (a) Müller, K.; Faeh, C.; Diederich, F. Science 2007, 317, 1881.
(b) Purser, S.; Moore, P. R.; Swallow, S.; Gouverneur, V. Chem. Soc. Rev. 2008, 37, 320.
(c) Zhou, Y.; Wang, J.; Gu, Z.; Wang, S.; Zhu, W.; Aceña, J. L.; Soloshonok, V. A.; Izawa, K.; Liu, H. Chem. Rev. 2016, 116, 422.
[3] Kirsch, P. Modern Fluoroorganic Chemistry:Synthesis, Reactivity, Applications, Wiley-VCH, Weinheim, Germany, 2013.
[4] (a) Zhang, C.-P.; Chen, Q.-Y.; Guo, Y.; Xiao, J.-C.; Gu, Y.-C. Chem. Soc. Rev. 2012, 41, 4536.
(b) Chu, L.; Qing, F.-L. Acc. Chem. Res. 2014, 47, 1513.
(c) Xu, X. H.; Matsuzaki, K.; Shibata, N. Chem. Rev. 2015, 115, 731.
(d) Ni, C.; Hu, M.; Hu, J. Chem. Rev. 2015, 115, 765.
(e) Liu, X.; Xu, C.; Wang, M.; Liu, Q. Chem. Rev. 2015, 115, 683.
[5] (a) Wang, X.; Zhang, Y.; Wang, J.-B. Sci. Sin. Chim. 2012, 42, 1417(in Chinese). (王兮, 张艳, 王剑波, 中国科学:化学, 2012, 42, 1417.)
(b) Pan, F.; Shi, Z. Acta Chim. Sinica 2012, 70, 1679. (潘菲, 施章杰, 化学学报, 2012, 70, 1679.)
(c) Qing, F.-L. Chin. J. Org. Chem. 2012, 32, 815(in Chinese). (卿凤翎, 有机化学, 2012, 32, 815.)
(d) Zeng, W.; Chen F. Chin. J. Appl. Chem. 2014, 31, 627(in Chinese). (曾薇, 陈甫雪, 应用化学, 2014, 31, 627.)
(e) Ma, J.-A.; Cahard, D. J. Fluorine Chem. 2007, 128, 975.
(f) Hui, R.; Zhang, S.; Tan, Z.; Wu, X.; Feng, B. Chin. J. Org. Chem. 2017, 37, 3060(in Chinese). (惠人杰, 张士伟, 谭政, 吴小培, 冯柏年, 有机化学, 2017, 37, 3060.)
(g) Gou, B.; Yang, C.; Zhang, L.; Xia, W. Acta Chim. Sinica 2017, 75, 66(in Chinese). (苟宝权, 杨超, 张磊, 夏吾炯, 化学学报, 2017, 75, 66.)
(h) Song, H.-X.; Han, Q.-Y.; Zhao, C.-L.; Zhang, C.-P. Green Chem. 2018, 20, 1662.
[6] (a) Dolbier, W. R. Chem. Rev. 1996, 96, 1557.
(b) Studer, A. Angew. Chem. Int. Ed. 2012, 51, 8950.
[7] (a) Haszeldine, R. N. J. Chem. Soc. 1949, 2856.
(b) Scherer, K. V.; Ono, T.; Yamanouchi, K.; Fernandez, R.; Henderson, P. J. Am. Chem. Soc. 1985, 107, 718.
(c) Umemoto, T.; Ando, A. Bull. Chem. Soc. Jpn. 1986, 59, 447.
(d) Sawada, H.; Nakayama, M.; Yoshida, M.; Yoshida, T.; Kamigata, N. J. Fluorine Chem. 1990, 46, 423.
(e) Langlois, B. R.; Laurent, E.; Roidot, N. Tetrahedron Lett. 1991, 32, 7525.
(f) Bertrand, F.; Pevere, V.; Quiclet-Sire, B.; Zard, S. Z. Org. Lett. 2001, 3, 1069.
(g) Fujiwara, Y.; Dixon, J. A.; O'Hara, F.; Funder, E. D.; Dixon, D. D.; Rodriguez, R. A.; Baxter, R. D.; Herle, B.; Sach, N.; Collins, M. R.; Ishihara, Y.; Baran, P. S. Nature 2012, 492, 95.
(h) Sato, A.; Han, J.; Ono, T.; Wzorek, A.; Acena, J. L.; Soloshonok, V. A. Chem. Commun. 2015, 51, 5967.
(i) Rong, J.; Deng, L.; Tan, P.; Ni, C.; Gu, Y.; Hu, J. Angew. Chem. Int. Ed. 2016, 55, 2743.
(j) Liu, P.; Liu, W.; Li, C. J. J. Am. Chem. Soc. 2017, 139, 14315.
(k) Rong, J.; Ni, C.; Wang, Y.; Kuang, C.; Gu, Y.; Hu, J. Acta Chim. Sinica 2017, 75, 105(in Chinese). (荣健, 倪传法, 王云泽, 匡翠文, 顾玉诚, 胡金波, 化学学报, 2017, 75, 105.)
(l) Daniel, M.; Dagousset, G.; Diter, P.; Klein, P. A.; Tuccio, B.; Goncalves, A. M.; Masson, G.; Magnier, E. Angew. Chem. Int. Ed. 2017, 56, 1.
(m) Ouyang, Y.; Xu, X. H.; Qing, F. L. Angew. Chem. Int. Ed. 2018, 57, 6926.
(n) Yang, B.; Yu, D.; Xu, X.-H.; Qing, F.-L. ACS Catal. 2018, 2839.
(o) Liu, Y.; Shao, X.; Zhang, P.; Lu, L.; Shen, Q. Org. Lett. 2015, 17, 2752.
[8] (a) Chatterjee, T.; Iqbal, N.; You, Y.; Cho, E. J. Acc. Chem. Res. 2016, 49, 2284.
(b) Koike, T.; Akita, M. Acc. Chem. Res. 2016, 49, 1937.
(c) Cho, E. J. Chem. Rec. 2016, 16, 47.
(d) Zeng, T.; Xuan, J.; Chen, J.; Lu, L.; Xiao, W. Imag. Sci. Photochem. 2014, 32, 415(in Chinese). (曾婷婷, 宣俊, 陈加荣, 陆良秋, 肖文精, 影像科学与光化学, 2014, 32, 415.)
[9] (a) Xu, J.; Liu, X.; Fu, Y. Tetrahedron Lett. 2014, 55, 585.
(b) Koike, T.; Akita, M. J. Fluorine Chem. 2014, 167, 30.
(c) Wang, S.-M.; Han, J.-B.; Zhang, C.-P.; Qin, H.-L.; Xiao, J.-C. Tetrahedron 2015, 71, 7949.
(d) Prieto, A.; Baudoin, O.; Bouyssi, D.; Monteiro, N. Chem. Commun. 2016, 52, 869.
(e) Ling, L.; Liu, K.; Li, X.; Li, Y. ACS Catal. 2015, 5, 2458.
(f) He, X.; Shan, C.; Qi, X.; Bai, R.; Lan, Y. Sci. Sin. Chim. 2017, 47, 859(in Chinese). (何晓倩, 单春晖, 戚孝天, 白若鹏, 蓝宇, 中国科学:化学, 2017, 47, 859.)
(g) Ye, J.-H.; Zhu, L.; Yan, S.-S.; Miao, M.; Zhang, X.-C.; Zhou, W.-J.; Li, J.; Lan, Y.; Yu, D.-G. ACS Catal. 2017, 7, 8324.;
(h) Zhu, L.; Ye, J.-H.; Duan, M.; Qi, X.; Yu, D.-G.; Bai, R.; Lan, Y. Org. Chem. Front. 2018, 5, 633.
[10] (a) Beale, T. M.; Chudzinski, M. G.; Sarwar, M. G.; Taylor, M. S. Chem. Soc. Rev. 2013, 42, 1667.
(b) Cavallo, G.; Metrangolo, P.; Milani, R.; Pilati, T.; Priimagi, A.; Resnati, G.; Terraneo, G. Chem. Rev. 2016, 116, 2478.
[11] (a) Mulliken, R. S. J. Am. Chem. Soc. 1950, 72, 600.
(b) Rosokha, S. V.; Kochi, J. K. Acc. Chem. Res. 2008, 41, 641.
(c) Lima, C. G. S.; Lima, T. d. M.; Duarte, M.; Jurberg, I. D.; Paixão, M. W. ACS Catal. 2016, 6, 1389.
(e) Postigo, A. Eur. J. Org. Chem. 2018, https://doi.org/10.1002/ejoc.201801079.
[12] Sladojevich, F.; McNeill, E.; Börgel, J.; Zheng, S.-L.; Ritter, T. Angew. Chem. Int. Ed. 2015, 54, 3712.
[13] (a) Sun, X.; Wang, W.; Li, Y.; Ma, J.; Yu, S. Org. Lett. 2016, 18, 4638.
(b) Sun, X.; Wang, W.; Ma, J.; Yu, S. Acta Chim. Sinica 2017, 75, 115(in Chinese). (孙晓阳, 王文敏, 马晶, 俞寿云, 化学学报, 2017, 75, 115.)
(c) Sun, X.; He, Y.; Yu, S. J. Photochem. Photobiol., A 2018, 355, 326.
[14] Wang, Y.; Wang, J.; Li, G.-X.; He, G.; Chen, G. Org. Lett. 2017, 19, 1442.
[15] (a) Cheng, Y.; Yu, S. Org. Lett. 2016, 18, 2962.
(b) Jiang, H.; He, Y.; Cheng, Y.; Yu, S. Org. Lett. 2017, 19, 1240.
[16] Cheng, Y.; Yuan, X.; Ma, J.; Yu, S. Chem. Eur. J. 2015, 21, 8355.
[17] (a) Jiang, Y. Y.; Yu, H. Z.; Fu, Y.; Liu, L. Sci. China Chem. 2015, 58, 673.
(b) Mizuta, S.; Verhoog, S.; Wang, X.; Shibata, N.; Gouverneur, V.; Meciebielle, M. J. Fluorine Chem. 2013, 155, 124.
(c) Li, M.; Wang, Y.; Xue, X. S.; Cheng, J. P. Asian J. Org. Chem. 2017, 6, 235.
[18] (a) Li, M.; Guo, J.; Xue, X. S.; Cheng, J. P. Org. Lett. 2016, 18, 264.
(b) Li, M.; Xue, X. S.; Guo, J.; Wang, Y.; Cheng, J. P. J. Org. Chem. 2016, 81, 3119.
(c) Xue, X. S.; Wang, Y.; Li, M.; Cheng, J. P. J. Org. Chem. 2016, 81, 4280.
(d) Yan, T.; Zhou, B.; Xue, X. S.; Cheng, J. P. J. Org. Chem. 2016, 81, 9006.
(e) Zhang, P.; Li, M.; Xue, X.-S.; Xu, C.; Zhao, Q.; Liu, Y.; Wang, H.; Guo, Y.; Lu, L.; Shen, Q. J. Org. Chem 2016, 81, 7486.
(f) Zhou, B.; Yan, T.; Xue, X. S.; Cheng, J. P. Org. Lett. 2016, 18, 6128.
(g) Li, M.; Xue, X.-S.; Cheng, J.-P. ACS Catal. 2017, 7, 7977.
(h) Li, M.; Zhou, B.; Xue, X.-S.; Cheng, J.-P. J. Org. Chem. 2017, 82, 8697.
(i) Yang, J. D.; Wang, Y.; Xue, X. S.; Cheng, J. P. J. Org. Chem. 2017, 82, 4129.
(j) Zhou, B.; Xue, X. S.; Cheng, J. P. Tetrahedron Lett. 2017, 58, 1287.
(k) Li, M.; Sang, Y.; Xue, X.-S.; Cheng, J.-P. J. Org. Chem. 2018, 83, 3333.
(l) Li, M.; Zheng, H.; Xue, X.-S.; Cheng, J.-P. Tetrahedron Lett. 2018, 59, 1278.
(m) Zhang, J.; Yang, J.-D.; Zheng, H.; Xue, X.-S.; Mayr, H.; Cheng, J.-P. Angew. Chem. Int. Ed. 2018, 57, 12690.
[19] (a) Zhao, Y.; Truhlar, D. G. Acc. Chem. Res. 2008, 41, 157.
(b) Klippenstein, S. J.; Pande, V. S.; Truhlar, D. G. J. Am. Chem. Soc. 2014, 136, 528.
[20] Marenich, A. V.; Cramer, C. J.; Truhlar, D. G. J. Phys. Chem. B 2009, 113, 6378.
[21] Frisch, M. J.; Trucks, G. W.; Schlegel, H. B.; Scuseria, G. E.; Robb, M. A.; Cheeseman, J. R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G. A.; Nakatsuji, H.; Caricato, M.; Li, X.; Hratchian, H. P.; Izmaylov, A. F.; Bloino, J.; Zheng, G.; Sonnenberg, J. L.; Hada, M.; Ehara, M.; Toyota, K.; Fukuda, R.; Hasegawa, J.; Ishida, M.; Nakajima, T.; Honda, Y.; Kitao, O.; Nakai, H.; Vreven, T.; Montgomery, J. A.; Jr., J. E. P.; Ogliaro, F.; Bearpark, M.; Heyd, J. J.; Brothers, E.; Kudin, K. N.; Staroverov, V. N.; Keith, T.; Kobayashi, R.; Normand, J.; Raghavachari, K.; Rendell, A.; Burant, J. C.; Iyengar, S. S.; Tomasi, J.; Cossi, M.; Rega, N.; Millam, J. M.; Klene, M.; Knox, J. E.; Cross, J. B.; Bakken, V.; Adamo, C.; Jaramillo, J.; Gomperts, R.; Stratmann, R. E.; Yazyev, O.; Austin, A. J.; Cammi, R.; Pomelli, C.; Ochterski, J. W.; Martin, R. L.; Morokuma, K.; Zakrzewski, V. G.; Voth, G. A.; Salvador, P.; Dannenberg, J. J.; Dapprich, S.; Daniels, A. D.; Farkas, O.; Foresman, J. B.; Ortiz, J. V.; Cioslowski, J.; Fox, D. J. Gaussian 09, Revision D. 01, Gaussian, Inc., Wallingford CT, 2013.
[22] Naumann, D.; Wilkes, B.; Kischkewitz, J. J. Fluorine Chem. 1985, 30, 73.
[23] Naumann, D.; Kischkewitz, J. J. Fluorine Chem. 1990, 46, 265.
[24] Haszeldine, R. N. J. Chem. Soc. 1949, 2856.
[25] Stefani, A. P.; Herk, L.; Szwarc, M. J. Am. Chem. Soc. 1961, 83, 4732.
[26] Akiyama, T.; Kato, K.; Kajitani, M.; Sakaguchi, Y.; Nakamura, J.; Hayashi, H.; Sugimori, A. Bull. Chem. Soc. Jpn. 1988, 61, 3531.
[27] Sangster, J. M.; Thynne, J. C. J. J. Phys. Chem. 1969, 73, 2746.
[28] Kamigata, N.; Fukushima, T.; Yoshida, M. J. Chem. Soc., Chem. Commun. 1989, 1559.
[29] Hu, L.-Q.; Huang, W.-Y. Chin. J. Chem. 1989, 9, 498(in Chinese). (胡里清, 黄维垣, 有机化学, 1989, 9, 498.)
[30] Billard, T.; Roques, N.; Langlois, B. R. J. Org. Chem. 1999, 64, 3813.
[31] Gong, J.; Fuchs, P. L. J. Am. Chem. Soc. 1996, 118, 4486.
[32] Lai, C.; Mallouk, T. E. J. Chem. Soc., Chem. Commun. 1993, 1359.
[33] Shi, G.; Shao, C.; Pan, S.; Yu, J.; Zhang, Y. Org. Lett. 2015, 17, 38.
[34] Beatty, J. W.; Douglas, J. J.; Cole, K. P.; Stephenson, C. R. J. Nat. Commun. 2015, 6, 1.
[35] Kawamura, S.; Sodeoka, M. Angew. Chem. Int. Ed. 2016, 55, 8740.
[36] Billard, T.; Roques, N.; Langlois, B. R. Tetrahedron Lett. 2000, 41, 3069.
[37] Umemoto, T. J. Fluorine Chem. 2014, 167, 3.
[38] (a) Gleiter, R.; Haberhauer, G.; Werz, D. B.; Rominger, F.; Bleiholder, C. Chem. Rev. 2018, 118, 2010.
(b) Vogel, L.; Wonner, P.; Huber, S. M. Angew. Chem. Int. Ed. 2018, DOI:10.1002/anie.201809432.
/
| 〈 |
|
〉 |