

Cu/Chiral Phosphoric Acid-Catalyzed Asymmetric Radical-Initiated Aminoarylation of Alkenes
Received date: 2018-10-07
Online published: 2018-10-15
Supported by
Project supported by the National Natural Science Foundation of China (Nos. 21722203, 21572096), the Shenzhen Special Funds (Nos. JCYJ20170412152435366, JCYJ20170307105638498) and the Shenzhen Nobel Prize Scientists Laboratory Project (No. C17213101).
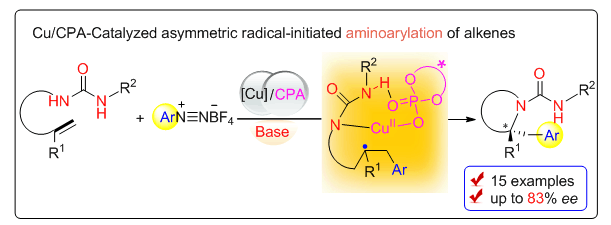
Enantioenriched pyrrolidines bearing a β-aryl group and an α-quaternary carbon stereocenter are important structural motifs in many natural products and pharmaceuticals, and their enantioselective synthesis has thus received extensive attention over the last several decades. Nonetheless, so far as we know, asymmetric aminoarylation of alkenes to access such targets has only been independently reported by Wolfe and Liu using palladium catalysis involving a key aminopalladation step, and thus, general and practical methodologies towards a variety of chiral pyrrolidines are still in demand and highly desirable. As part of our ongoing interest in radical-initiated difunctionalization reactions of alkenes based on Cu(I)/chiral phosphoric acid (CPA) catalysis, we sought to develop a mechanistically distinct and complementary approach for this asymmetric palladium(Ⅱ)-catalyzed aminoarylation of alkenes. Herein we describe our efforts toward the development of the efficient asymmetric radical-initiated aminoarylation of alkenes with aryldiazonium salts enabled by Cu(I)/CPA catalysis. A general procedure for the aminoarylation of alkenes with aryldiazonium salts is as follows:under argon, an oven-dried resealable Schlenk tube equipped with a magnetic stir bar was charged with urea substrate 1 (0.1 mmol, 1.0 equiv.), CuI (1.9 mg, 0.01 mmol, 10 mol%), CPA[(S)-A1 (9.3 mg, 0.015 mmol, 15 mol%], aryldiazonium salts 2 (0.12 mmol, 1.2 equiv.), Na3PO4 (19.7 mg, 0.12 mmol, 1.2 equiv.) and isopropyl acetate(1.0 mL) at 32℃ and the sealed tube was then stirred under the same conditions. Upon completion (monitored by thin-layer chromatography), the reaction mixture was directly purified by silica gel chromatography[eluent:V(petroleum ether):V(EtOAc)=100:0 to 3:1] to afford the desired product 3. The enantiometric excess of product was determined by chiral high-performance liquid chromatography (HPLC) analysis. A broad scope of substrates worked well under this standard conditions to afford enantioenriched pyrrolidines in good yield with good to excellent enantioselectivity. A series of control experiments were conducted to determine the reaction mechanism as a radical process and a possible mechanism was proposed.
Li Xue-Fei , Lin Jin-Shun , Wang Jian , Li Zhong-Liang , Gu Qiang-Shuai , Liu Xin-Yuan . Cu/Chiral Phosphoric Acid-Catalyzed Asymmetric Radical-Initiated Aminoarylation of Alkenes[J]. Acta Chimica Sinica, 2018 , 76(11) : 878 -882 . DOI: 10.6023/A18100413
[1] (a) Royles, B. J. L. Chem. Rev. 1995, 95, 1981;
(b) O'Hagan, D. Nat. Prod. Rep. 2000, 17, 435.
(c) Lewis, J. R. Nat. Prod. Rep. 2001, 18, 95.
(d) Fish, P. V.; Andrews, M. D.; Jonathan Fray, M.; Stobie, A.; Wakenhut, F.; Whitlock, G. A. Bioorg. Med. Chem. Lett. 2009, 19, 2829.
[2] (a) Fache, F.; Schulz, E.; Tommasino, M. L.; Lemaire, M. Chem. Rev. 2000, 100, 2159.
(b) Notz, W.; Tanaka, F.; Barbas, C. F. Acc. Chem. Res. 2004, 37, 580.
(c) Erkkila, A.; Majander, I.; Pihko, P. M. Chem. Rev. 2007, 107, 5416.
(d) Caputo, C. A.; Jones, N. D. Dalton Trans. 2007, 4627.
[3] (a) Pettit, G. R.; Goswami, A.; Cragg, G. M.; Schmidt, J. M.; Zou, J. C. J. Nat. Prod. 1984, 47, 913.
(b) Su, B.; Cai, C.; Deng, M.; Wang, Q. J. Agric. Food. Chem. 2016, 64, 2039.
[4] Oloff, S.; Mailman, R. B.; Tropsha, A. J. Med. Chem. 2005, 48, 7322.
[5] Burnett, D. A.; Hart, D. J. J. Org. Chem. 1987, 52, 5662.
[6] Lebon, F.; Pegurier, C.; Ledecq, M.; Mathieu, B.; Bosman, N.; Frycia, A.; Lengele, S.; Dhurke, K.; Kanduluru, A. K.; Meunier, S.; Wagner, A.; Wolff, C.; Provins, L. Bioorg. Med. Chem. Lett. 2012, 22, 3978.
[7] For selected reviews, see:(a) Coldham, I.; Hufton, R. Chem. Rev. 2005, 105, 2765.
(b) Pandey, G.; Banerjee, P.; Gadre, S. R. Chem. Rev. 2006, 106, 4484.
(c) Pellissier, H. Tetrahedron 2007, 63, 3235.
(d) Nicolle, S. M.; Lewis, W.; Hayes, C. J.; Moody, C. J. Angew. Chem., Int. Ed. 2016, 55, 3749.
[8] For selected racemic examples, see:(a) Ney, J. E.; Wolfe, J. P. Angew. Chem., Int. Ed. 2004, 43, 3605.
(b) Ney, J. E.; Hay, M. B.; Yang, Q.; Wolfe, J. P. Adv. Synth. Catal. 2005, 347, 1614.
(c) Bertrand, M. B.; Leathen, M. L.; Wolfe, J. P. Org. Lett. 2007, 9, 457.
(d) Bertrand, M. B.; Neukom, J. D.; Wolfe, J. P. J. Org. Chem. 2008, 73, 8851.
(e) Brenzovich, W. E., Jr.; Benitez, D.; Lackner, A. D.; Shunatona, H. P.; Tkatchouk, E.; Goddard, W. A., 3rd; Toste, F. D. Angew. Chem., Int. Ed. 2010, 49, 5519.
(f) Tkatchouk, E.; Mankad, N. P.; Benitez, D.; Goddard, W. A., 3rd; Toste, F. D. J. Am. Chem. Soc. 2011, 133, 14293.
(g) Fumagalli, G.; Boyd, S.; Greaney, M. F. Org. Lett. 2013, 15, 4398.
(h) Sahoo, B.; Hopkinson, M. N.; Glorius, F. J. Am. Chem. Soc. 2013, 135, 5505.
(i) Fornwald, R. M.; Fritz, J. A.; Wolfe, J. P. Chem.-Eur. J. 2014, 20, 8782.
(j) Alicea, J.; Wolfe, J. P. J. Org. Chem. 2014, 79, 4212.
(k) Faulkner, A.; Scott, J. S.; Bower,
J. F. J. Am. Chem. Soc. 2015, 137, 7224.
(l) Peterson, L. J.; Wolfe, J. P. Adv. Synth. Catal. 2015, 357, 2339.
(m) Garad, D. N.; Mhaske, S. B. Org. Lett. 2016, 18, 3862.
(n) Yang, H.-B.; Pathipati, S. R.; Selander, N. ACS Catal. 2017, 7, 8441.
(o) Yang, M.-N.; Yan, D.-M.; Zhao, Q.-Q.; Chen, J.-R.; Xiao, W.-J. Org. Lett. 2017, 19, 5208.
(p) Zhang, R.; Xu, Q.; Shi, M. Acta Chim. Sinica 2012, 70, 1593. (张睿, 徐琴, 施敏, 化学学报, 2012, 70, 1593.)
(q) Zhang, J.; Wu, Z.; Xie, F.; Zhang, W. Chin. J. Org. Chem. 2018, 38, 1319. (张金刚, 吴正兴, 谢芳, 张万斌, 有机化学, 2018, 38, 1319.)
[9] For selected reviews, see:(a) Heinrich, M. R. Chem. Eur. J. 2009, 15, 820.
(b) Hari, D. P.; Koenig, B. Angew. Chem., Int. Ed. 2013, 52, 4734.
(c) Kindt, S.; Heinrich, M. R. Synthesis 2016, 48, 1597.
[10] For selected examples, see:(a) Sahoo, B.; Hopkinson, M. N.; Glorius, F. J. Am. Chem. Soc. 2013, 135, 5505.
(b) Hari, D. P.; Hering, T.; Koenig, B. Angew. Chem., Int. Ed. 2014, 53, 725.
(c) Kindt, S.; Wicht, K.; Heinrich, M. R. Org. Lett. 2015, 17, 6122.
[11] (a) Mai, D. N.; Wolfe, J. P. J. Am. Chem. Soc. 2010, 132, 12157.
(b) Babij, N. R.; Wolfe, J. P. Angew. Chem., Int. Ed. 2013, 52, 9247.
(c) Hopkins, B. A.; Wolfe, J. P. Chem. Sci. 2014, 5, 4840.
(d) White, D. R.; Hutt, J. T.; Wolfe, J. P. J. Am. Chem. Soc. 2015, 137, 11246.
(e) Garlets, Z. J.; Parenti, K. R.; Wolfe, J. P. Chem.-Eur. J. 2016, 22, 5919.
[12] Zhang, W.; Chen, P.; Liu, G. Angew. Chem., Int. Ed. 2017, 56, 5336.
[13] (a) Neukom, J. D.; Perch, N. S.; Wolfe, J. P. J. Am. Chem. Soc. 2010, 132, 6276.
(b) Neukom, J. D.; Perch, N. S.; Wolfe, J. P. Organometallics 2011, 30, 1269.
[14] Zhu, R.; Buchwald, S. L. J. Am. Chem. Soc. 2015, 137, 8069.
[15] Lin, J.-S.; Dong, X.-Y.; Li, T.-T.; Jiang, N.-C.; Tan, B.; Liu, X.-Y. J. Am. Chem. Soc. 2016, 138, 9357.
[16] Lin, J.-S.; Wang, F.-L.; Dong, X.-Y.; He, W.-W.; Yuan, Y.; Chen, S.; Liu, X.-Y. Nat. Commun. 2017, 8, 14841.
[17] Wang, F.-L.; Dong, X.-Y.; Lin, J.-S.; Zeng, Y.; Jiao, G.-Y.; Gu, Q.-S.; Guo, X.-Q.; Ma, C.-L.; Liu, X.-Y. Chem 2017, 3, 979.
[18] (a) Wang, F.; Wang, D.; Wan, X.; Wu, L.; Chen, P.; Liu, G. J. Am. Chem. Soc. 2016, 138, 15547.
(b) Wang, D.; Wang, F.; Chen, P.; Lin, Z.; Liu, G. Angew. Chem., Int. Ed. 2017, 56, 2054.
(c) Wu, L.; Wang, F.; Wan, X.; Wang, D.; Chen, P.; Liu, G. J. Am. Chem. Soc. 2017, 139, 2904.
[19] Cheng, Y.-F.; Dong, X.-Y.; Gu, Q.-S.; Yu, Z.-L.; Liu, X.-Y. Angew. Chem., Int. Ed. 2017, 56, 8883.
/
| 〈 |
|
〉 |