

Influence of C—F…H—X Interactions on Organic Reactions
Received date: 2018-08-31
Online published: 2018-10-18
Supported by
Project supported by the National Natural Science Foundation of China (Nos. 21472049, 81660576) and Guizhou engineering research center for the exploitation and utilization technology of medicine and food dual-use resources.
Although the debate on whether or not C―F bonds can function as H-bond acceptors lasted for tens of years, dating back to 1939 when Pauling pointed out in The Nature of the Chemical Bond that C-F bonds do not have significant power to act as proton acceptors in the formation of hydrogen bonds, more and more evidences support the existence of C―F…H―X interactions, and in particular, C―F…H―O and C―F…H―N interactions cannot be ignored.Because the sum of the van der Waals radii of hydrogen and fluorine atoms is reported to be around as 2.55 Å, C―F…H―X interactions may exist if the calculated distance of F…H is less than 2.50 Å. Strong C―F…H―X interactions may occur if the calculated distance is less than 2.30 Å and the F…H―X angle is greater than 120°.In 2011, we observed strong fluorine effects on the Strecker reaction of ketimines: while Schreiner's thiourea could catalyze the Strecker reaction of acetophenone derived ketimine using TMSCN, it was unable to mediate the corresponding reaction of analogy α-CF3 or α-CF2H ketimines. Theoretical calculations revealed that the C―F…H―N interactions between the C―F bond of fluorinated ketimines and thiourea played the key role. This is the first report on the influence of such subtle interactions on organic reactions. Since then, reports from our and other groups revealed various types of C―F…H―X interactions that may be present in the reaction course, to strongly influence the reactivity and selectivity. Although successful examples are still limited, these achievements have suggested that C―F…H―X interactions may exist between the substrate and the catalyst; the substrate and the solvent; different reaction partners, or engender in the transition state with the reaction intermediate. Importantly, known examples demonstrate it possible to harness C―F…H―X interactions to tune reactivity and/or selectivity, which are useful for new reaction development, as well as for the design of new catalysts. To provide reference and inspiration for researchers engaged in organic synthesis, especially the organic fluorine chemistry, we summarize in this review the recent advances in the study of the influences of C―F…H―X interactions on organic reactions.
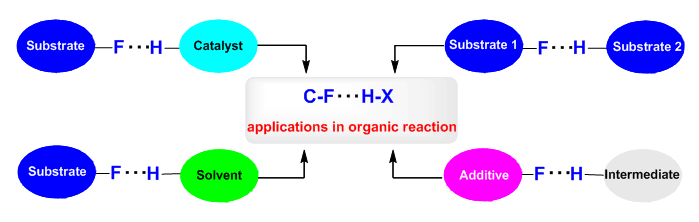
Key words: C―F…H―X interaction; organic reaction; reactivity; selectivity
Hao Yong-Jia , Yu Jin-Sheng , Zhou Ying , Wang Xin , Zhou Jian . Influence of C—F…H—X Interactions on Organic Reactions[J]. Acta Chimica Sinica, 2018 , 76(12) : 925 -939 . DOI: 10.6023/A18080360
[1] Arunan, E.; Desiraju, G. R.; Klein, R. A.; Sadlej, J.; Scheiner, S.; Alkorta, I.; Clary, D. C.; Crabtree, R. H.; Dannenberg, J. J.; Hobza, P.; Kjaergaard, H. G.; Legon, A. C.; Mennucci, B.; Nesbitt, D. J. Pure Appl. Chem. 2011, 83, 1619.
[2] Desiraju, G. R. Angew. Chem., Int. Ed. 2011, 50, 52.
[3] Latimer, W. M.; Rodebush, W. H. J. Am. Chem. Soc. 1920, 42, 1419.
[4] Robertson, J. M. Nature 1935, 136, 755.
[5] Taylor, R.; Kennard, O. J. Am. Chem. Soc. 1982, 104, 5063.
[6] Katz, B. A.; Spencer, J. R.; Elrod, K.; Luong, C.; Mackman, R. L.; Rice, M.; Sprengeler, P. A.; Allen, D.; Janc, J. J. Am. Chem. Soc. 2002, 124, 11657.
[7] (a) Bondar, A. N.; White, S. H. BBA-Biomembranes 2012, 1818, 942.
(b) Zhang, J.; Chen, P. C.; Yuan, B. K.; Ji, W.; Cheng, Z. H.; Qiu, X. H. Science 2013, 342, 611.
[8] For the recent works on H-bonding interaction from Chinese research group:(a) Wang, M.; Cheng, C. Q.; Song, J. T.; Wang, J.; Zhou, X. G.; Xiang, H. F.; Liu, J. Chin. J. Chem. 2018, 36, 698.
(b) Zhu, X. W.; Cui, X. Y.; Cai, W. S.; Shao, X. G. Acta Chim. Sinica 2018, 76, 298(in Chinese). (朱雪薇, 崔晓宇, 蔡文生, 邵学广, 化学学报, 2018, 76, 298.).
(c) Sun, G. J.; Nie, C. B.; Zhao, X.; Li, Z. T. Chin. J. Org. Chem. 2017, 37, 1757(in Chinese). (孙广军, 聂承斌, 赵新, 黎占亭, 有机化学, 2017, 37, 1757.)
[9] Desiraju, G. R. Acc. Chem. Res. 1991, 24, 290.
[10] Jeffrey, G. A. Cryst. Rev. 1995, 4, 213.
[11] For a review on H-bond donor catalysis, see:Doyle, A. G.; Jacobsen, E. N. Chem. Rev. 2007, 107, 5713. For bifucntional catalysis with H-bond donors, see amide based organocatalysts:(a) Liu, X. H.; Lin, L. L.; Feng, X. M. Chem. Commun. 2009, 41, 6145; with enamine catalysis:(b) Kano, T.; Maruoka, K. Chem. Commun. 2008, 43, 5465; with tertiary phosphine catalysis:(c) Wei, Y.; Shi, M. Acc. Chem. Res. 2010, 43, 1005;
(d) Xu, L. W. ChemCatChem 2013, 5, 2775;
(e) Wang, S. X.; Han, X. Y.; Zhong, F. R.; Wang, Y. Q.; Lu, Y. X. Synlett 2011, 19, 2766; with NHCs catalysis:(f) Grossmann, A.; Enders, D. Angew. Chem., Int. Ed. 2012, 51, 314;
(g) Sun, L. H.; Liang, Z. Q.; Jia, W. Q.; Ye, S. Angew. Chem., Int. Ed., 2013, 52, 5803;
(h) Lv, H.; Jia, W. Q.; Sun, L. H.; Ye, S. Angew. Chem., Int. Ed. 2013, 52, 8607; with PTC catalysis:(i) Novacek, J.; Waser, M. Eur. J. Org. Chem. 2013, 4, 637;
(j) Shirakawa, S.; Maruoka, K. Tetrahedron Lett. 2014, 55, 3833; with metal catalysis:(k) Song, J.; Guo, C.; Chen, P. H.; Yu, J.; Luo, S. W.; Gong, L. Z. Chem. Eur. J. 2011, 17, 7786;
(l) Lang, K.; Park, J.; Hong, S. Angew. Chem., Int. Ed. 2012, 51, 1620. with phosphoramide:(m) Ding, M.; Zhou, F.; Liu, Y. L.; Wang, C. H.; Zhao, X. L.; Zhou, J. Chem. Sci. 2011, 2, 2035.
(n) Gao, W. M.; Yu, J. S.; Zhao, Y. L.; Liu, Y. L.; Zhou, F.; Wu, H. H.; Zhou, J. Chem. Commun. 2014, 50, 15179.
[12] O'Hagan, D. Chem. Soc. Rev. 2008, 37, 308.
[13] Howard, J. A. K.; Hoy, V. J.; O'Hagan, D.; Smith, G. T. Tetrahedron 1996, 52, 12613.
[14] Pauling, L. The Nature of the Chemical Bond, Cornell University Press, Ithaca, NY, 1939, p. 28.
[15] Dunitz, J. D.; Taylor, R. Chem. Eur. J. 1997, 3, 89.
[16] Shimoni, L.; Glusker, J. P. Struct. Chem. 1994, 5, 383.
[17] Thalladi, V. R.; Weiss, H. C.; Bla1ser, D.; Boese, R.; Nangia, A.; Desiraju, G. R. J. Am. Chem. Soc. 1998, 120, 8702.
[18] Thakur, T. S.; Kirchner, M. T.; Bläser, D.; Boese, R.; Desiraju, G. R. CrystEngComm 2010, 12, 2079.
[19] Anzahaee, M. Y.; Watts, J. K.; Alla, N. R.; Nicholson, A. W.; Damha, M. J. J. Am. Chem. Soc. 2011, 133, 728.
[20] Schneider, H. J. Chem. Sci. 2012, 3, 1381.
[21] Zhao, X.; Wang, X. Z.; Jiang, X. K.; Chen, Y. Q.; Li, Z. T.; Chen, G. J. J. Am. Chem. Soc. 2003, 125, 15128.
[22] Liu, Y. L.; Shi, T. D.; Zhou, F.; Zhao, X. L.; Wang, X.; Zhou, J. Org. Lett. 2011, 13, 3826.
[23] (a) Pesenti, C.; Viani, F. ChemBioChem 2004, 5, 590.
(b) Müller, K.; Faeh, C.; Diederich, F. Science 2007, 317, 1881.
(c) Hagmann, W. K. J. Med. Chem. 2008, 51, 4359.
(d) Wang, J.; Liu, H. Chin. J. Org. Chem. 2011, 31, 1785(in Chinese). (王江, 柳红, 有机化学, 2011, 31, 1785.)
(e) Ojima, I. J. Org. Chem. 2014, 44, 6358.
(f) Liu, Y. L.; Yu, J. S.; Zhou, J. Asian J. Org. Chem. 2013, 2, 194.
(g) Lin, J. H.; Xiao, J. C. Tetrahedron Lett. 2014, 55, 6147.
(h) Ni, C. F.; Zhu, L. G.; Hu, J. B. Acta Chim. Sinica 2015, 73, 90(in Chinese). (倪传法, 朱林桂, 胡金波, 化学学报, 2015, 73, 90.)
(i) Champagne, P. A.; Desroches, J.; Hamel, J. D.; Vandamme, M.; Paquin, J. F. Chem. Rev. 2015, 115, 9073.
(j) Zhang, J.; Jin, C. F.; Zhang, Y. J. Chin. J. Org. Chem. 2014, 34, 662(in Chinese). (张霁, 金传飞, 张英俊, 有机化学, 2014, 34, 662.)
(k) Swallow, S. Progress in Medicinal Chemistry 2015, 54, 65.
(l) Gillis, E. P.; Eastman, K. J.; Hill, M. D.; Donnelly, D. J.; Meanwell, N. A. J. Med. Chem. 2015, 58, 8315.
(m) Wang, G. M.; Zhu, Z. D.; Chen, Z. Q.; Xu, Z. J.; Zhu, W. L. Acta Pharmaceutica Sinica 2018, 53, 701(in Chinese). (王桂敏, 朱正诞, 陈照强, 徐志建, 朱维良, 药学学报, 2018, 53, 701.)
(n) Ni, C. F.; Hu, J. B. Chem. Soc. Rev. 2016, 45, 5441.
(o) Cahard, D.; Bizet, V. Chem. Soc. Rev. 2014, 43, 135.
(p) Fustero, S.; Fuentes, A. S.; Barrio, P.; Haufe, G. Chem. Rev. 2015, 115, 871.
[24] Champagne, P. A.; Desroches, J.; Paquin, J. F. Synthesis 2014, 47, 306.
[25] Vachal, P.; Jacobsen, E. N. J. Am. Chem. Soc. 2002, 124, 10012.
[26] Jin, L. M.; Xu, X.; Lu, H. J.; Cui, X.; Wojtas, L.; Zhang, X. P. Angew. Chem., Int. Ed. 2013, 52, 5309.
[27] Yuan, H. N.; Wang, S.; Nie, J.; Meng, W.; Yao, Q.; Ma, J. A. Angew. Chem., Int. Ed. 2013, 52, 3869.
[28] Lee, K. A.; Silverio, D. L.; Torker, S.; Robbins, D. W.; Haeffner, F.; Mei, F. W.; Hoveyda, A. H. Nat. Chem. 2016, 8, 768.
[29] (a) Liu, Y. L.; Zeng, X. P.; Zhou, J. Chem. Asian J. 2012, 7, 1759.
(b) Liu, H. X.; Tao, Z.; Xie, Q.; Zhou, J.; Wang, X. Comput. Theor. Chem. 2018, 1142, 57.
(c) Liu, Y. L.; Zhou, F.; Cao, J. J.; Ji, C. B.; Ding, M.; Zhou, J. Org. Biomol. Chem. 2010, 8, 3847.
(d) Ji, C. B.; Cao, Z. Y.; Wang, X.; Wu, D. Y.; Zhou, J. Chem. Asian J. 2013, 8, 877.
[30] For the related proton-transfer process, see:(a) Xia, Y. Z.; Liang, Y.; Chen, Y. Y.; Wang, M.; Jiao, L.; Huang, F.; Liu, S.; Li, Y. H.; Yu, Z. X. J. Am. Chem. Soc. 2007, 129, 3470.
(b) Shi, F. Q.; Li, X.; Xia, Y. Z.; Zhang, L. M.; Yu, Z. X. J. Am. Chem. Soc. 2007, 129, 15503.
(c) Li, X.; Ye, S. Y.; He, C.; Yu, Z. X. Eur. J. Org. Chem. 2008, 25, 4296.
[31] Champagne, P. A.; Pomarole, J.; Thérien, M. È.; Benhassine, Y.; Beaulieu, S.; Legault, C. Y.; Paquin, J. F. Org. Lett. 2013, 15, 2210.
[32] Champagne, P. A.; Benhassine, Y.; Desroches, J.; Paquin, J. F. Angew. Chem., Int. Ed. 2014, 53, 13835.
[33] Rosenberg, R. E. J. Phys. Chem. A 2012, 116, 10842.
[34] Dalvit, C.; Invernizzi, C.; Vulpetti, A. Chem. Eur. J. 2014, 20, 11058.
[35] For our efforts in selective fluoroalkylation using fluorinated enol silyl ethers, see:(a) Liu, Y. L.; Zhou, J. Chem. Commun. 2012, 48, 1919.
(b) Liu, Y. L.; Zhou, J. Acta Chim. Sinica 2012, 70, 1451(in Chinese). (刘运林, 周剑, 化学学报, 2012, 70, 1451.)
(c) Liu, Y. L.; Liao, F. M.; Niu, Y. F.; Zhao, X. L.; Zhou, J. Org. Chem. Front. 2014, 1, 742.
(d) Liao, F. M.; Liu, Y. L.; Yu, J. S.; Zhou, F.; Zhou, J. Org. Biomol. Chem. 2015, 13, 8906.
(e) Yu, J. S.; Zhou, J. Org. Biomol. Chem. 2015, 13, 10968.
(f) Yu, J. S.; Liao, F. M.; Gao, W. M.; Liao, K.; Zuo, R. L.; Zhou, J. Angew. Chem., Int. Ed. 2015, 54, 7381.
(g) Yu, J. S.; Zhou, J. Org. Chem. Front. 2016, 3, 298.
(h) Zeng, X. P.; Zhou, J. J. Am. Chem. Soc. 2016, 138, 8730.
(i) Liao, F. M.; Cao, Z. Y.; Yu, J. S.; Zhou, J. Angew. Chem., Int. Ed. 2017, 56, 2459.
(j) Hu, X. S.; Du, Y.; Yu, J. S.; Liao, F. M.; Ding, P. G.; Zhou, J. Synlett 2017, 28, 2194.
(k) Liao, F. M.; Gao, X. T.; Hu, X. S.; Xie, S. L.; Yu, J. S.; Zhou, J. Sci. Bull. 2017, 62, 1504.
(l) Liao, F. M.; Du, Y.; Zhou, F.; Zhou, J. Acta Chim. Sinica 2018, 76, DOI:10.6023/A18060238(in Chinese). (廖富民, 杜溢, 周锋, 周剑, 化学学报, 2018, 76, DOI:10.6023/A18060238).
[36] Yu, J. S.; Liu, Y. L.; Tang, J.; Wang, X.; Zhou, J. Angew. Chem., Int. Ed. 2014, 53, 9512.
[37] For the use of chiral Lewis base to activate silyl reagents, see:(a) Tian, S. K.; Deng, L. J. Am. Chem. Soc. 2001, 123, 6195.
(b) Tian, S. K.; Hong, R.; Deng, L. J. Am. Chem. Soc. 2003, 125, 9900.
(c) Fuerst, D. E.; Jacobsen, E. N. J. Am. Chem. Soc. 2005, 127, 8964.
(d) Wang, J.; Hu, X. L.; Jiang, J.; Gou, S. H.; Huang, X.; Liu, X. H.; Feng, X. M. Angew. Chem., Int. Ed. 2007, 46, 8468.
(e) Wang, J.; Wang, W. T.; Li, W.; Hu, X. L.; Shen, K.; Tan, C.; Liu, X. H.; Feng, X. M. Chem. Eur. J. 2009, 15, 11642.
(f) Liu, Y. L.; Zhou, J. Chem. Commun. 2013, 49, 4421.
(g) Zhao, Y. L.; Cao, Z. Y.; Zeng, X. P.; Shi, J. M.; Yu, Y. H.; Zhou, J. Chem. Commun. 2016, 52, 3943. For a review:Liu, Y. L.; Zhou, J. Synthesis 2015, 47, 1210.
[38] Aronoff, M. R.; Gold, B.; Raines, R. T. Tetrahedron Lett. 2016, 57, 2347.
[39] Aronoff, M. R.; Gold, B.; Raines, R. T. Org. Lett. 2016, 18, 1538.
[40] Lou, H. Q.; Wang, Y. T.; Jin, E. Z.; Lin, X. F. J. Org. Chem. 2016, 81, 2019.
[41] Liu, Y. B.; Hu, L. R.; Chen, H.; Du, H. F. Chem. Eur. J. 2015, 21, 3495.
[42] (a) Wang, X. M.; Han, Z. B.; Wang, Z.; Ding, K. L. Angew. Chem., Int. Ed. 2012, 51, 936.
(b) Wang, X. M.; Meng, F. Y.; Wang, Y.; Han, Z. B.; Chen, Y. J.; Liu, L.; Wang, Z.; Ding, K. L. Angew. Chem., Int. Ed. 2012, 51, 9276.
[43] (a) Cao, Z. Y.; Wang, X. M.; Tan, C.; Zhao, X. L.; Zhou, J.; Ding, K. L. J. Am. Chem. Soc. 2013, 135, 8197. Also see:(b) Cao, Z. Y.; Zhou, F.; Zhou, J. Acc. Chem. Res. 2018, 61, 1443.
(c) Cao, Z. Y.; Zhou, J. Org. Chem. Front. 2015, 2, 849.
[44] Cao, Z. Y.; Wang, W. M.; Liao, K.; Wang, X.; Zhou, J.; Ma, J. Org. Chem. Front. 2018, 5, 2960.
[45] We also reported a Hg-catalyzed cyclopropanation of diazooxindoles with alkenes, and found that the N-methyl group of diazooxindole had no negative influence on enantioselectivity, possibly because the reaction solvent is not fluorinated solvents, see:Cao, Z. Y.; Zhou, F.; Yu, Y. H.; Zhou, J. Org. Lett. 2013, 15, 42.
[46] (a) Ess, D. H.; Houk, K. N. J. Am. Chem. Soc. 2007, 129, 10646.
(b) van Zeist, W. J.; Bickelhaupt, F. M. Org. Biomol. Chem. 2010, 8, 3118.
(c) Bickelhaupt, F. M.; Houk, K. N. Angew. Chem., Int. Ed. 2017, 56, 10070.
/
| 〈 |
|
〉 |