

Preparation and Preliminary Molecular Imaging Study of 124I in-situ Labeled Organic Melanin Nanoparticles
Received date: 2018-09-29
Online published: 2018-11-01
Supported by
Project supported by the National Natural Science Foundation of China (Nos. 81671733, 81501519 and 81571705) and Beijing Nova program (No. Z171100001117020).
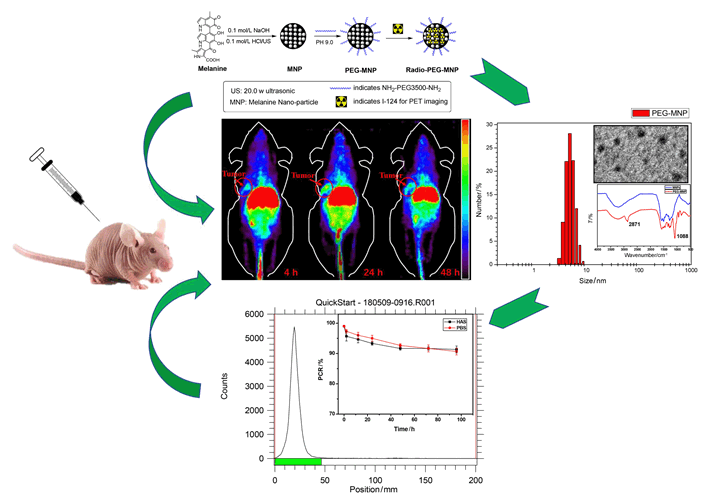
Developing biocompatible, multifunctional and in-situ labeling nanoplatform is high challenging for molecular imaging. Organic derivates melanin nanoparticles (MNPs) holds great potential to be multimodal contrast agents, and have been used for photoacoustic imaging, magnetic resonance imaging, and 64Cu PET imaging with simple modifications. In order to extend MNPs application in molecular imaging, here a novel radio-nuclide was applied to in-situ labeling of MNPs. Large numbers of active dihydroxyindole/indolequinone groups and natural binding ability of MNPs enabled them to have the ability to label different types of radionuclides which have unique half-life and functions, especially long-life elemental nuclide. This project explored the in-situ labeling methods of organic melanin nanoparticles with a promising diagnostic radionuclides named Iodine-124 (124I), and using this novel multifunctional organic nanoparticles for preliminary molecular imaging studies. Generally, ultrafine particle size melanin nanoparticles (5.5 nm in diameter) were prepared by ultrasonication method using naturally occurring melanin, then PEG3500 which had amino group at both ends was used as a stabilizer agent to obtain PEG-MNP nanocarriers (7.5 nm in diameter) with better water solubility and stability. The nanoparticles were full characterized by dynamic light scattering (DLS), transmission electron microscope (TEM) and 1H NMR, respectively. Then, one kind of elemental nuclide was labeled. Classic iodine labeled method with N-Bromo Succinimide (NBS) was used as oxidant to oxidize active dihydroxyindole/indolequinone ring of PEG-MNP for electrophilic substitution reaction labeling 124I (100.8 h). This reaction rate is extremely fast (60 s reaction time) and high labelling yield (>99%). The 124I was labeled successfully and in-situ labeled PEG-MNP nanocarriers were obtained. After that, 124I and 124I-PEG-MNP were used to further preclinical evaluation by micro-PET imaging. Micro-PET images were collected at 2 h, 24 h and 48 h after intravenous injection 7.4 MBq 124I and 124I-PEG-MNP in normal Kunming mice (n=3). The ROI target area of heart, liver and thyroid were delineated for semi-quantitative analysis. Then, in order to verify the imaging ability of 124I-PEG-MNP in solid tumor. We built human pancreatic cancer BxPC3 xenograft model (n=3), and Micro-PET scans were performed at different time points. Results showed that the labeling rate of 124I on PEG-MNP was 99.9%. And the radiochemical purity in vitro stability of 124I-PEG-MNP in 96 h was more than 90%. Micro-PET images showed that 124I-PEG-MNP had no obvious thyroid uptake which indicated no de-marking in mice. The radio-distribution of 124I and 124I-PEG-MNP was substantially different in liver and thyroid (P<0.001). In vivo semi-quantitative analysis showed that the radio uptakes of organs were consistent with the distribution of nanoparticles. And the PET imaging of xenograft mice showed that 124I-PEG-MNP can utilize the enhanced permeability and retention effect (EPR) to be significantly enriched at the tumor and retained in the tumor site for more than 48 h. PEG-MNP has the ability to label long half-life nuclide 124I. This research provides an experimental basis for further construction of long-circulation multimodal imaging probes.
Xia Lei , Cheng Zhen , Zhu Hua , Yang Zhi . Preparation and Preliminary Molecular Imaging Study of 124I in-situ Labeled Organic Melanin Nanoparticles[J]. Acta Chimica Sinica, 2019 , 77(2) : 172 -178 . DOI: 10.6023/A18090410
[1] Ding, L.; Liu, Z.; Aggrey, M. O.; Li, C.; Chen, J.; Tong, L. Mini Rev. Med. Chem. 2015, 15, 529.
[2] Liu, M. L.; Wu, Q.; Shi, H. F.; An, Z. F.; Huang, W. Acta Chim. Sinica 2018, 76, 246(in Chinese). (刘明丽, 吴琪, 史慧芳, 安众福, 黄维, 化学学报, 2018, 76, 246.)
[3] Xu, M. M.; Guo, C.; Hu, G.; Xu, S.; Wang, L. Chinese J. Chem. 2018, 36, 25.
[4] Sun, Y.; Hong, S.; Ma, X.; Cheng, K.; Wang, J.; Zhang, Z.; Yang, M.; Jiang, Y.; Hong, X.; Cheng, Z. Chem. Sci. 2016, 7, 5888.
[5] Castellanos, G.; Fernández-Seara, M. A.; Lorenzo-Betancor, O.; Ortega-Cubero, S.; Puigvert, M.; Uranga, J.; Vidorreta, M.; Irigoyen, J.; Lorenzo, E.; Muñoz-Barrutia, A.; Ortiz-de-Solorzano, C.; Pastor, P.; Pastor, M. A. Mov. Disord. 2015, 30, 945.
[6] Liu, Y.; Ai, K.; Liu, J.; Deng, M.; He, Y.; Lu, L. Adv. Mater. 2013, 25, 1353.
[7] Hong, S. H.; Sun, Y.; Tang, C.; Cheng, K.; Zhang, R.; Fan, Q.; Xu, L.; Huang, D.; Zhao, A.; Cheng, Z. Bioconjugate Chem. 2017, 28, 1925.
[8] Ha, S. W.; Cho, H. S.; Yoon, Y. I.; Jang, M. S.; Hong, K. S.; Hui, E.; Lee, J. H.; Yoon, T. J. J. Nanobiotechnology 2017, 15, 73.
[9] Li, Y.; Xie, Y.; Wang, Z.; Zang, N.; Carniato, F.; Huang, Y.; Andolina, C. M.; Parent, L. R.; Ditri, T. B.; Walter, E. D.; Botta, M.; Rinehart, J. D.; Gianneschi, N. C. ACS Nano 2016, 10, 10186.
[10] Xu, W.; Sun, J.; Li, L.; Peng, X.; Zhang, R.; Wang, B. Biomater. Sci. 2017, 19, 207.
[11] Zhu, H.; Li, N.; Lin, X. F.; Hong, Y.; Yang, Z. Acta Chim. Sinica 2014, 72, 427(in Chinese). (朱华, 李囡, 林新峰, 洪业, 杨志, 化学学报, 2014, 72, 427.)
[12] Bosch, P.; Sucunza, D.; Mendicuti, F.; Domingo, A.; Vaquero, J. Org. Chem. Front. 2018, 5, 1916.
[13] Yang, M. P.; Su, N.; Li, Y. X.; Wang, L.; Ma, L. F.; Zhang, Y.; Li, J.; Yang, B. Q.; Kang, L. L. Chinese J. Org. Chem. 2018, 38, 636.
[14] Wang, Y. J.; Wang, W. Acta Chim. Sinica 2017, 75, 1061. (王咏婕, 王伟, 化学学报, 2017, 75, 1061.)
[15] Fan, Q.; Cheng, K.; Hu, X.; Ma, X.; Zhang, R.; Yang, M.; Lu, X.; Xing, L.; Huang, W.; Gambhir, S. S.; Cheng, Z. J. Am. Chem. Soc. 2014, 136, 15185.
[16] Xie, Q.; Zhu, H.; Wang, F.; Meng, X. X.; Ren, Q. S.; Xia, C. Q.; Yang, Z. Molecules 2017, 22, 641.
[17] Hoshyar, N.; Gray, S.; Han, H.; Bao, G. Nanomedicine 2016, 11, 673.
[18] Lubberink, M.; Herzog, H. Eur. J. Nucl. Med. Mol. Imaging 2011, 38, 10.
[19] Zhang, R.; Fan, Q.; Yang, M.; Cheng, K.; Lu, X.; Zhang, L.; Huang, W.; Cheng, Z. Adv. Mater. 2015, 27, 5063.
[20] Choi, C. H.; Zuckerman, J. E.; Webster, P.; Davis, M. E. Proc. Natl. Acad. Sci. U. S. A. 2011, 108, 6656.
/
| 〈 |
|
〉 |