

N—H and O—H Difluoromethylation of N-Heterocycles
Received date: 2018-07-11
Online published: 2018-11-08
Supported by
Project supported by the National Natural Science Foundation of China (No. 21772046), the Recruitment Program of Global Experts and the Natural Science Foundation of Fujian Province (No. 2016J01064).
It is known that fluorine is the strongest in electronegativity and a peculiar element. Fluorinated compounds are extensively applied in the areas of pharmaceuticals, agrochemical, materials, life sciences, etc., due to the unique chemical, physical and biological properties of fluorine-containing compounds. Therefore, the development of expedient synthetic strategies for the introduction of —F, —CF2H and —CF3 into organic compounds has attracted much attentions of chemists. Although trifluoromethylation has been well developed, difluoromethylation has been less reported. We found that difluorocarbene (:CF2) could be generated in situ from ethyl bromodifluoroacetate (BrCF2COOEt) in the presence of Na2CO3, which could go through N—H, O—H difluoromethylation smoothly. The scope of substrates was broad, and various functional groups, such as halogen, formyl group, nitro-group, nitrile and so on could be tolerated well. This would be a potential and practical reaction in modification of various bioactive drugs beause benzimidazole, indazole and pyridine are the skeleton of medicine and nature molecule. In addition, a representative procedure for this reaction is as following: An oven-dried Schlenk tube (10 mL) was equipped with a magnetic stir bar, the substrates of nitrogen-containing or oxygen-containing (0.3 mmol), the base (Na2CO3,2 equiv., 0.6 mmol), ethyl bromodifluoroacetate (1.2 equiv., 0.36 mmol). The flask was evacuated and backfilled with N2 for 3 times, acetone or acetonitrile as a solvent for 24 h under N2 atmosphere. Where after the solvent concentrated in vacuo and the residue was purified by chromatography on silica gel with ethyl acetate:petroleum ether (EA:PE=1:30) to afford the corresponding products.
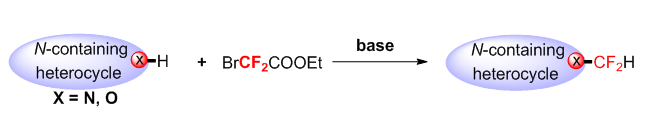
Ma Xingxing , Xuan Qingqing , Song Qiuling . N—H and O—H Difluoromethylation of N-Heterocycles[J]. Acta Chimica Sinica, 2018 , 76(12) : 972 -976 . DOI: 10.6023/A18070265
[1] (a) Müller, K.; Faeh, C.; Diederich, F. Science 2007, 317, 1881;
(b) Furuya, T.; Kamlet, A. S.; Ritter, T. Nature 2011, 473, 470;
(c) Hiyama, T. Organofluorine Compounds Chemistry and Applications, Springer-Verlag, Berlin Heidelberg, 2000;
(d) O'Hagan, D. Chem. Soc. Rev. 2008, 37, 308;
(e) Purser, S.; Moore, P. R.; Swallow, S.; Gouverneur, V. Chem. Soc. Rev. 2008, 37, 320;
(e) Wang, J.; Sanchez-Rosello, M.; Acen, J. L.; del Pozo, C.; Sorochinsky, A. E.; Fustero, S.; Soloshonok, V. A.; Liu, H. Chem. Rev. 2014, 114, 2432;
(f) Brahms, D.; Dailey, W. Chem. Rev. 1996, 96, 1585;
(g) Dolbier, W.; Battiste, M. Chem. Rev. 2003, 103, 1071;
(h) Fedoryński, M. Chem. Rev. 2003, 103, 1099;
(i) Ed.:Hiyama, T., Organofluorine Compounds:Chemistry and Applications, Springer, New York, 2000;
(j) Hong, M.; Min, J.; Wang, S. Chin. J. Org. Chem. 2018, 38, 1907. (洪梅, 闵洁, 王石发, 有机化学, 2018, 38, 1907.)
[2] (a) Erickson, J. A.; McLoughlin, J. I. J. Org. Chem. 1995, 60, 1626;
(b) Meanwell, N. A. J. Med. Chem. 2011, 54, 2529;
(c) Kirk, K. L. Org. Process Res. Dev. 2008, 12, 305;
(d) Prakash, G. K. S.; Chacko, S. Curr. Opin. Drug Discovery Dev. 2008, 11, 793;
(e) Feng, Z.; Min, Q.-Q.; Fu, X-P.; An, L.; Zhang, X. Nature Chem. 2017, 9, 918;
(f) Ge, S.; Chaladaj, W.; Hartwig, J. F. J. Am. Chem. Soc. 2014, 136, 4149;
(g) Zafrani, Y.; Yeffet, D.; Sod-Moriah, G.; Berliner, A.; Amir, D.; Marciano, D.; Gershonov, E.; Saphier, S. J. Med. Chem. 2017, 60, 797;
(h) Xu, C.; Guo, W-H.; He, X.; G, Y-L.; Zhang, X.-Y.; Zhang, X. Nat. Commun. 2018, 9, 1170;
(i) Gu, Y.; Leng, X.; Shen, Q. Nat. Commun. 2014, 5, 5405;
(j) Ran, Y.; Lin, Q.-Y.; Xu, X.-H.; Qing, F.-L. J. Org. Chem. 2016, 81, 7001.
[3] (a) Hu, J.; Zhang, W.; Wang, F. Chem. Commun. 2009, 7465;
(b) Hu, J.; Ni, C. Synthesis 2014, 46, 842;
(c) Leo, A.; Hansch, C.; Elkins, D. Chem. Rev. 1971, 71, 525;
(d) Hansch, C.; Leo, A.; Taft, R.W. Chem. Rev. 1991, 91, 165;
(e) Leroux, F.; Jeschke, P.; Schlosser, M. Chem. Rev. 2005, 105, 827;
(f) Manteau, B.; Pazenok, S.; Vors, J. P.; Leroux, F. R. J. Fluorine Chem. 2010, 131, 140.
(g) Rong, J.; Ni, C.; Wang, Y.; Kuang, C.; Gu, Y.; Hu, J. Acta Chim. Sinica 2017, 75, 105. (荣健, 倪传法, 王云泽, 匡翠文, 顾玉诚, 胡金波, 化学学报, 2017, 75, 105);
(h) Sun, X.; Wang, W.; Ma, J.; Yu, S. Acta Chim. Sinica 2017, 75, 115. (孙晓阳, 王文敏, 马晶, 俞寿云, 化学学报, 2017, 75, 115);
(i) Zhou, N.; Xu, P.; Li, W.; Cheng, Y.; Zhu, C. Acta Chim. Sinica 2017, 75, 60. (周能能, 胥攀, 李伟鹏, 成义祥, 朱成建, 化学学报, 2017, 75, 60);
(j) Zhang, L.; Wu, B.; Chen, Z.; Hu, J. Chin. J. Org. Chem. 2018, 38, 2028. (章吕烨, 吴彬强, 陈张涛, 胡锦锦, 曾晓飞, 钟国富, 有机化学, 2018, 38, 2028);
(k) Chen, C.; Fu, L.; Chen, P.; Liu, G. Chin. J. Chem. 2017, 35, 1781.
[4] (a) Furuya, T.; Kamlet, A. S.; Ritter, T. Nature 2011, 473, 470;
(b) Tomashenko, O. A.; Grushin, V. V. Chem. Rev. 2011, 111, 4475;
(c) Besset, T.; Schneider, C.; Cahard, D. Angew. Chem., Int. Ed. 2012, 51, 5048;
(d) Ni, C.; Hu, M.; Hu, J. Chem. Rev. 2015, 115, 765;
(e) Ni, C.; Zhu, L.; Hu, J. Acta Chim. Sinica 2015, 73, 90. (倪传法, 朱林桂, 胡金波, 化学学报, 2015, 73, 90);
(f) Zhang, P.; Lu, L.; Shen, Q. Acta Chim. Sinica 2017, 75, 744. (张盼盼, 吕龙, 沈其龙, 化学学报, 2017, 75, 744);
(g) Chachignon, H.; Cahard, D. Chin. J. Chem. 2016, 34, 445.
[5] (a) Xie, Q.; Ni, C.; Zhang, R.; Li, L.; Rong, J.; Hu, J. Angew. Chem., Int. Ed. 2017, 56, 3206;
(b) Li, L.; Wang, F.; Ni, C.; Hu, J. Angew. Chem., Int. Ed. 2013, 52, 12390;
(c) Gu, J-W.; Zhang, X. Org. Lett. 2015, 17, 5384;
(d) Flynn, R. M.; Burton, D. J. J. Fluorine Chem. 2011, 132, 815;
(e) Feng, Z.; Min, Q.-Q.; Zhang, X. Org. Lett. 2016, 18, 44;
(f) Ke, M.; Song, Q. J. Org. Chem. 2016, 81, 3654;
(g) Ke, M.; Song, Q. Chem. Commun. 2017, 53, 2222;
(h) Ke, M.; Song, Q. Adv. Synth. Catal. 2017, 359, 384;
(i) Ke, M.; Feng, Q.; Yang, K.; Song, Q. Org. Chem. Front. 2016, 3, 150;
(j) Taguchi, T.; Kitagawa, O.; Morikawa, T.; Nishiwaki, T.; Uehara, H.; Endo, H.; Kobayashi, Y. Tetrahedron Lett. 1986, 27, 6103;
(k) Araki, K.; Inoue, M. Tetrahedron 2013, 69, 3913;
(l) Belhomme, M.-C.; Poisson, T.; Pannecoucke, X. Org. Lett. 2013, 15, 3428;
(m) Feng, Z.; Min, Q.-Q.; Fu, X-P.; An, L.; Zhang, X. Nature Chem. 2017, 9, 918.
[6] (a) Burton, D. J.; Wiemers, D. M. J. Am. Chem. Soc. 1985, 107, 5014;
(b) Wiemers, D. M.; Burton, D. J. J. Am. Chem. Soc. 1986, 108, 832;
(c) Zheng, J.; Lin, J.-H.; Deng, X.-Y.; Xiao, J.-C. Org. Lett. 2015, 17, 532;
(d) Brooks, A. F.; Topczewski, J. J.; Ichiishi, N.; Sanford, M. S.; Scott, P. J. H. Chem. Sci. 2014, 5, 4545;
(e) Huiban, M.; Tredwell, M.; Mizuta, S.; Wan, Z.; Zhang, X.; Collier, T. L.; Gouverneur, V.; Passchier, J. Nat. Chem. 2013, 5, 941;
(f) van der Born, D.; Sewing, C.; Herscheid, J. D. M.; Windhorst, A. D.; Orru, R. V. A.; Vugts, D. J. Angew. Chem., Int. Ed. 2014, 53, 11046;
(g) Ivashkin, P.; Lemonnier, G.; Cousin, J.; Grégoire, V.; Labar, D.; Jubault, P.; Pannecoucke, X. Chem.-Eur. J. 2014, 20, 9514;
(h) Ruhl, T.; Rafique, W.; Lien, V. T.; Riss, P. J. Chem. Commun. 2014, 50, 6056.
[7] See reviews for the formation of:CF2H (a) Fu, X.-P.; Xiao, Y.-L.; Zhang, X. Chin. J. Chem. 2018, 36, 143;
(b) Zhang, W.; Zhu, L.; Hu, J. Tetrahedron 2007, 63, 10569. See reviews for difluoro-cyclopro-panes:(c) Toshiyuki, I. Current Fluoroorganic Chemistry, Vol. 949, ACS Symposium Series, American Chemical Society, 2007, pp. 352-362;
(d) Burch, J. D.; Barrett, K.; Chen, Y.; DeVoss, J.; Eigenbrot, C.; Goldsmith, R.; Ismaili, M. H. A.; Lau, K.; Lin, Z.; Ortwine, D. F.; Zarrin, A. A.; McEwan, P. A.; Barker, J. J.; Ellebrandt, C.; Kordt, D.; Stein, D. B.; Wang, X.; Chen, Y.; Hu, B.; Xu, X.; Yuen, P.-W.; Zhang, Y.; Pei, Z. J. Med. Chem. 2015, 58, 3806;
(e) Dantzig, A. H.; Shepard, R. L.; Law, K. L.; Tabas, L.; Pratt, S.; Gillespie, J. S.; Binkley, S. N.; Kuhfeld, M. T.; Starling, J. J.; Wrighton, S. A. J. Pharmacol. Exp. Ther. 1999, 290, 854;
(f) Itoh, T.; Kanbara, M.; Ohashi, M.; Hayase, S.; Kawatsura, M.; Kato, T.; Miyazawa, K.; Takagi, Y.; Uno, H. J. Fluorine Chem. 2007, 128, 1112.
[8] (a) Fuchibe, K.; Aono, T.; Hu, J.; Ichikawa, J. Org. Lett. 2016, 18, 4502; see reviews for[3+2] and[2+2+1] cyclizations:(b) Coscia, R. W.; Lambert, T. H. J. Am. Chem. Soc. 2009, 131, 2496 and refs therein;
(c) Lautens, M.; Klute, W.; Tam, W. Chem. Rev. 1996, 96, 49;
(d) Frü hauf, H.-W. Chem. Rev. 1997, 97, 523; see reviews for Junji Ichikawa's previous works about transition metal difluorocarbene complexes:(e) Aono, T.; Sasagawa, H.; Fuchibe, K.; Ichikawa, J. Org. Lett. 2015, 17, 5736.
[9] Imidazole derivatives play an important role in chemical and biological systems, see reviews:(a) Bando, T.; Sugiyama, H. Acc. Chem. Res. 2006, 39, 935;
(b) Breslow, R. Acc. Chem. Res. 1991, 24, 317;
(c) Townsend, L. B. Chem. Rev. 1967, 67, 533;
(d) Palui, G.; Aldeek, F.; Wang, W.; Mattoussi, H. Chem. Soc. Rev. 2015, 44, 193.
[10] (a) Ma, X.; Zhou, Y.; Song, Q. Org. Lett. 2018, 20, 4777;
(b) Ma, X.; Mai, S.; Zhou, Y.; Cheng, G.-J.; Song, Q. Chem. Commun. 2018, 54, 8960.
/
| 〈 |
|
〉 |