

B(C6F5)3-Catalyzed Chemoselective Reduction of Carbonyl Compounds under Water Conditions
Received date: 2018-10-11
Online published: 2018-11-08
Supported by
Project supported by the National Natural Science Foundation of China (No. 21542011), and Scientific Research Fund of Leshan Normal University (Z1414, Z1308).
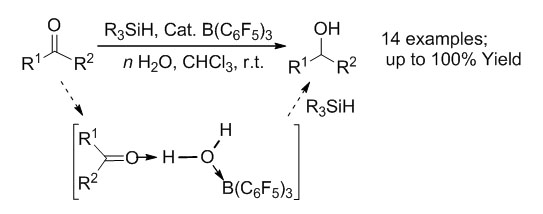
Recently, the research work concerning B(C6F5)3 catalyzed reduction of carbonyl compounds revealed that this Lewis acid B(C6F5)3 presents, actually, a rather water-tolerant system. This fact considerably broadens the scope of the water/base tolerant frustrated Lewis pairs (FLP) chemistry. In this research, an efficient chemoselective reduction of aldehydes and ketones to alcohols catalyzed by the Lewis acid B(C6F5)3 has been developed. It is the first report about the chemoselective reduction of carbonyl compounds under aqueous conditions catalyzed by FLPs with hydridosilanes as reducing agents. The selectivity and activity of different hydridosilanes and the influence of substituents in carbonyl compounds have been studied. The effect of water concentration on the chemoselectivity of the reaction has also been investigated. It has been found that a 2~3 fold excess of water relatively to hydridosilanes usually exhibits better selectivity and overall yields than in the equimolar case. The reduction reaction can even be successfully performed with pure water as a solvent without any loss of the reactivity. Such a procedure has been successfully applied to reduce 14 differently substituted aldehydes and ketones into alcohols with up to 100% yields under mild conditions, but failed in case of the diaryl substituted ketones. Both experimental and computational methods have been performed to confirm the possibility of the water mediated mechanism and the effects of different Lewis bases on the LB——H-OH——LA three-component aggregates. These mechanistic studies have revealed that such water mediation between a carbonyl compound and a catalyst advantageously (i) activates the C=O group by protonation and (ii) fixes the catalytic borane moiety by formation of a B-O bond, which to some extent prevents the direct hydrolysis of hydridosilane and makes the reaction possible under moist conditions. Detailed clarification of the actual role of water in the reduction reaction of question will promote the further development of FLP-catalyzed and related reactions in the "green" chemistry field.
Sun Guofeng , He Yunqing , Tian Chong , Borzov Maxim , Hu Qishan , Nie Wanli . B(C6F5)3-Catalyzed Chemoselective Reduction of Carbonyl Compounds under Water Conditions[J]. Acta Chimica Sinica, 2019 , 77(2) : 166 -171 . DOI: 10.6023/A18100423
[1] de Vries, J. G.; Elsevier, C. J. The Handbook of Homogeneous Hydrogenation, Wiley-VCH, Weinheim, Germany, 2008.
[2] Sorella, G. L.; Sperni, L.; Canton, P.; Coletti, L.; Fabris, F.; Strukul, G.; Scarso, A. J. Org. Chem. 2018, 83(14), 7438.
[3] Leischner, T.; Spannenberg, A.; Junge, K.; Beller, M. Organometallics 2018, DOI:10.1021/acs.organomet.8b00410.
[4] Cao, Y.; Ma, R.; Wang, N.; Wang, M.-Y.; Li, X.-D.; He, L.-N. J. CO2 Util. 2018, 24, 328.
[5] Call, A.; Lloret-Fillol, J. Chem. Commun. 2018, 54, 9643.
[6] Zhang, J.; Qu, L.; Shi, G.; Liu, J.; Chen, J.; Dai, L. Angew. Chem., Int. Ed. 2016, 55, 2230.
[7] Chakraborty, S.; Bhattacharya, P.; Dai, H.; Guan, H. Acc. Chem. Res. 2015, 48, 1995.
[8] Guo, J.; Chen, J.; Lu, Z. Chem. Commun. 2015, 51, 5725.
[9] Dai, L.; Xue, Y.; Qu, L.; Choi, H. J.; Baek, J. B. Chem. Rev. 2015, 115, 4823.
[10] Mahdi, T.; Stephan, D. W. Angew. Chem., Int. Ed. 2015, 54, 8511.
[11] Volkov, A.; Gustafson, K. P. J.; Tai, C. W.; Verho, O.; Baeckvall, J. E.; Adolfsson, H. Angew. Chem., Int. Ed. 2015, 54, 5122.
[12] Parks, D. J.; Piers, W. E. J. Am. Chem. Soc. 1996, 118, 9440.
[13] Stephan, D. W.; Erker, G. Angew. Chem., Int. Ed. 2010, 49, 46.
[14] Liu, Y.-B.; Du, H.-F. Acta Chim. Sinica 2014, 72, 771(in Chinese). (刘勇兵, 杜海峰, 化学学报, 2014, 72, 771.)
[15] Xuan, Q.; Zhao, C.; Song, Q. Org. Biomol. Chem. 2017, 15, 5140.
[16] Wei, S.; Du, H. J. Am. Chem. Soc. 2014, 136, 12261.
[17] Oestreich, M.; Hermeke, J.; Mohr, J. Chem. Soc. Rev. 2015, 44, 2202.
[18] Stephan, D. W.; Erker, G. Angew. Chem., Int. Ed. 2015, 54, 6400.
[19] Ren, X.; Du, H. J. Am. Chem. Soc. 2016, 38, 810.
[20] Stephan, D. W.; Greenberg, S.; Graham, T. W.; Chase, P.; Hastie, J. J.; Geier, S. J.; Farrell, J. M.; Brown, C. C.; Heiden, Z. M.; Welch, G. C.; Ullrich, M. Inorg. Chem. 2011, 50, 12338.
[21] Mahdi, T. Stephan, D. W. J. Am. Chem. Soc. 2014, 136, 15809.
[22] Scott, D. J.; Fuchter, M. J.; Ashley, A. E. J. Am. Chem. Soc. 2014, 136, 15813.
[23] Scott, D. J.; Simmons, T. R.; Lawrence, E. J.; Wildgoose, G. G.; Fuchter, M. J.; Ashley, A. E. ACS Catal. 2015, 5, 5540.
[24] Gyömöre, A.; Bakos, M.; Földes, T.; Papai, I.; Domja, N. A.; Soós, T. ACS Catal. 2015, 5, 5366.
[25] Parks, D. J.; Blackwell, J. M.; Piers, W. E. J. Org. Chem. 2000, 65, 3090.
[26] Piers, W. E.; Marwitz, A. J. V.; Mercier, L. G. Inorg. Chem. 2011, 50, 12252.
[27] Nie, W.-L.; Klare, H. F. T.; Oestreich, M.; Froehlich, R.; Kehr, G.; Erker, G. Z. Naturforsch. 2012, 67b, 987.
[28] Ermeke, J.; Mewald, M.; Oestreich, M. J. Am. Chem. Soc. 2013, 135, 17537.
[29] Houghton, A. Y.; Hurmalainen, J.; Mansikkamaeki, A.; Piers, W. E.; Tuononen, H. M. Nat. Chem. 2014, 6, 983.
[30] Nimmagadda, R. D.; McRae, C. Tetrahedron Lett. 2006, 47, 5755.
[31] Chatterjee, I.; Porwal, D.; Oestreich, M. Angew. Chem., Int. Ed. 2017, 56, 3389.
[32] Yang, W.-Y.; Gao, L.; Lu, J.; Song, Z.-L. Chem. Commun. 2018, 54, 4834.
[33] Parks, D. J.; Piers, W. E. J. Am. Chem. Soc. 1996, 118, 9440.
[34] Blackwell, J. M.; Foster, K. L.; Beck, V. H.; Piers, W. E. J. Org. Chem. 1999, 64, 4887.
[35] Tahara, A.; Sunada, Y.; Takeshita, T.; Inoue, R.; Nagashima, H. Chem. Commun. 2018, 54, 11192.
[36] Hu, X.; Tian, C.; Borzov, M.; Nie, W.-L. Acta Chim. Sinica, 2015, 73, 1025(in Chinese). (胡茜, 田冲, Borzov Maxim, 聂万丽, 化学学报, 2015, 73, 1025.)
[37] Tian, C.; Jiang, Y.; Borzov, M.; Nie, W.-L. Acta Chim. Sinica 2015, 73, 1203(in Chinese). (田冲, 姜亚, Borzov Maxim, 聂万丽, 化学学报, 2015, 73, 1203).
[38] Wen, Z.-G.; Tian, C.; Borzov, M.; Nie, W.-L. Acta Chim. Sinica 2016, 74, 498(in Chinese). (温志国, 田冲, Borzov Maxim, 聂万丽, 化学学报, 2016, 74, 498).
[39] Nie, W.-L.; Sun, G.-F.; Tian, C.; Borzov, M. Z. Naturforsch. 2016, 71(10)b, 1029.
[40] Zhang, L.-W.; Wen, Z.-G.; Borzov, M.; Nie, W.-L. Acta Chim. Sinica 2017, 75, 819(in Chinese). (张露文, 温志国, Borzov Maxim, 聂万丽, 化学学报, 2017, 75, 819).
[41] Fasano, V.; Radcliffe, J. E.; Ingleson, M. J. ACS Catal. 2016, 6(3), 1793.
[42] Fasano, V.; Ingleson, M. J. Chem. Eur. J. 2017, 23(9), 2217.
[43] Sun, G.-F.; Su, M.; Fang, J.; Borzov, M.; Nie, W.-L. Acta Chim. Sinica 2017, 75, 824(in Chinese). (孙国峰, 苏敏, 方洁, Borzov Maxim, 聂万丽, 化学学报, 2017, 75, 824).
[44] He, Y.-Q.; Teng, J. W.; Tian, C.; Borzov, M.; Hu, Q. S.; Nie, W.-L. Acta Chim. Sinica 2018, 76, 774(in Chinese). (何云清, 腾金伟, 田冲, Borzov Maxim, 胡启山, 聂万丽, 化学学报, 2018, 76, 774).
[45] He, Y.-Q.; Zou, M.-Y.; Xue, Y.; Hu, Q.-S.; Borzov, M. V.; Nie, W.-L. Mechanism Aspects of the B(C6F5)3 Catalyzed Reductive Amination, Chem-Eur. J. 2018, Submitted.
[46] Bergamaschi, G.; Lascialfari, L.; Pizzi, A.; Espinoza, M. I. M.; Demitri, N.; Milani, A.; Gori, A.; Metrangolo, P. Chem. Commun. 2018, DOI:10.1039/C8CC06010.
[47] Beck, A. D. J. Chem. Phys. 1993, 98, 5648.
[48] Parr, R. G.; Yang, W. Density Functional Theory of Atoms and Molecules, Oxford University Press, Oxford, 1989.
[49] Frisch, M. J.; Trucks, G. W.; Schlegel, H. B.; Scuseria, G. E.; Robb, M. A.; Cheeseman, J. R.; Scalmani, G.; Barone, V.; Petersson, G. A.; Nakatsuji, H.; Li, X.; Caricato, M.; Marenich, A. V.; Bloino, J.; Janesko, B. G.; Gomperts, R.; Mennucci, B.; Hratchian, H. P.; Ortiz, J. V.; Izmaylov, A. F.; Sonnenberg, J. L.; Williams-Young, D.; Ding, F.; Lipparini, F.; Egidi, F.; Goings, J.; Peng, B.; Petrone, A.; Henderson, T.; Ranasinghe, D.; Zakrzewski, V. G.; Gao, J.; Rega, N.; Zheng, G.; Liang, W.; Hada, M.; Ehara, M.; Toyota, K.; Fukuda, R.; Hasegawa, J.; Ishida, M.; Nakajima, T.; Honda, Y.; Kitao, O. Nakai, H. Vreven, T. Throssell, K. Montgomery, J. A.; Peralta, J. E.; Ogliaro, F.; Bearpark, M. J.; Heyd, J. J.; Brothers, E. N.; Kudin, K. N.; Staroverov, V. N.; Keith, T. A.; Kobayashi, R.; Normand, J.; Raghavachari, K.; Rendell, A. P.; Burant, J. C.; Iyengar, S. S.; Tomasi, J.; Cossi, M.; Millam, J. M.; Klene, M.; Adamo, C.; Cammi, R.; Ochterski, J. W.; Martin, R. L.; Morokuma, K.; Farkas, O.; Foresman, J. B.; Fox, D. J. Gaussian 16, Revision A.03, Gaussian, Inc., Wallingford CT, 2016.
[50] The typical 11B NMR signal of[R3NH] [HO-B(C6F5)3] is located at δ-3.9; the corresponding 19F NMR signals of o-, p-and m-F in[HO-B(C6F5)3] are at δ -135.6, -160.1, -164.8, respectively.
[51] Bergquist, C.; Bridgewater, B. M.; Harlan, C. J.; Norton, J. R.; Friesner, R. A.; Parkin, G. J. Am. Chem. Soc. 2000, 122, 10581.
[52] Di Saverio, A.; Focante, F.; Camurati, I.; Resconi, L.; Beringhelli, T.; D'Alfonso, G.; Donghi, D.; Maggioni, D.; Mercandelli, P.; Sironi, A. Inorg. Chem. 2005, 44, 5030.
/
| 〈 |
|
〉 |