

Nickel-Catalyzed Suzuki-Type Cross Coupling of Fluorinated Alkenyl Boronates with Alkyl Halides
Received date: 2018-08-13
Online published: 2018-11-09
Supported by
Project supported by the National Natural Science Foundation of China (Nos. 21572212, 21732006, 21702200, 51821006), and Major Program of Development Foundation of Hefei Center for Physical Science and Technology (2017FXZY001).
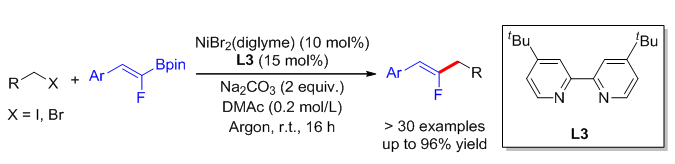
The incorporation of fluorine atoms or fluorine-containing fragments to specifical sites of organic compounds would result in unique diversifications in biological or physical properties, such as, significantly regulate the lipid solubility or metabolic stability, and promote specific binding ability to biological targets of target compounds. Monofluoroalkenes are ideal amide bond mimics, and have been widely used in the research field of pharmaceutical chemistry and drug discovery. Previously, we reported the nickel-catalyzed reductive cross coupling of gem-difluoroalkenes with unactivated secondary alkyl iodides and tertiary alkyl bromides. However, only medium yield can be obtained with primary alkyl halides, which might be caused by the lower stability and nucleophilic activity of these substrates. Herein, we report the nickel-catalyzed Suzuki-type cross coupling of fluorinated alkenyl boronates with alkyl halides for the synthesis of primary alkyl group substituted monofluoroalkenes. By using NiBr2(diglyme) (10 mol%) and 4,4'-di-tert-butyl-2,2'-bipyridine (15 mol%) as catalytic systems, Na2CO3 (2 equiv.) as base, N,N-dimethylacetamide as solvent, we achieved the cross coupling of a variety of fluorinated alkenyl boronates with primary alkyl iodides (e.g., 5), bromides (e.g., 9) and relatively inert secondary alkyl bromide (20). Under the mild reaction conditions, this reaction performed smoothly with good isolated yields and well functional group toleration. Many synthetically useful functional groups could survive during the transformation, such as, ether (6, 7), trifluoromethyl (8), cyano (10), ester (11), and even unprotected alcohol hydroxyl group (13). In addition, heterocycles such as tetrahydrofuran (14), phthalimide (15), dioxane (16), indole (17), pyridine (27) and quinoline (35) also posed no problem for this reaction. It should be pointed out that, this reaction is applicable not only to non-activated alkyl halides, but also to the conversion of activated allyl bromides (18, 19). For the fluorinated alkenyl boronates, this reaction also exhibited good functional group compatibility and wide substrate scope, and conducted successfully with both electron-rich (e.g., 4, 24), electron-neutral (e.g., 21), or electron-deficient (e.g., 27, 31) aromatic rings. Finally, the toleration of aryl sulfonate (30) provided further opportunities for subsequent modification through transition-metal-catalyzed cross coupling reactions. Radical clock experiment with (Z)-8-iodooct-3-ene (36) provided a mixture of linear product (37a) and ring-cyclized product (37b). (Bromomethyl)cyclopropane (38) was also subjected to the standard reaction conditions, only ring-opening product (39a) was obtained. In addition, this reaction was significantly inhibited with the addition of TEMPO (2,2,6,6-tetramethylpiperidinooxy). These results indicated a radical-type reaction mechanism for the cross coupling of fluorinated alkenyl boronates with alkyl halides. Further efforts would be devoted to develop one-pot synthesis of monofluoroalkenes through in-situ borylation of gem-difluoroalkenes and subsequent Suzuki-type cross coupling with alkyl halides.
He Shijiang , Pi Jingjing , Li Yan , Lu Xi , Fu Yao . Nickel-Catalyzed Suzuki-Type Cross Coupling of Fluorinated Alkenyl Boronates with Alkyl Halides[J]. Acta Chimica Sinica, 2018 , 76(12) : 956 -961 . DOI: 10.6023/A18080333
[1] (a) Gao, B.; Zhao, Y.; Hu, J. Angew. Chem., Int. Ed. 2015, 54, 638.
(b) Wu, X.; Xie, F.; Gridnev, I. D.; Zhang, W. Org. Lett. 2018, 20, 1638.
(c) Wang, M.; Pu, X.; Zhao, Y.; Wang, P.; Li, Z.; Zhu, C.; Shi, Z. J. Am. Chem. Soc. 2018, 140, 9061.
(d) Li, G.; Wang, T.; Fei, F.; Su, Y.-M.; Li, Y.; Lan, Q.; Wang, X.-S. Angew. Chem., Int. Ed. 2016, 55, 3491.
(e) Gong, T.-J.; Xu, M.-Y.; Yu, S.-H.; Yu, C.-G.; Su, W.; Lu, X.; Xiao, B.; Fu, Y. Org. Lett. 2018, 20, 570.
(f) Zheng, J.; Cai, J.; Lin, J.-H.; Guo, Y.; Xiao, J.-C. Chem. Commun. 2013, 49, 7513.
(g) Sha, M.; Zhang, D.; Pan, R.; Xing, P.; Jiang, B. Acta Chim. Sinica 2015, 73, 395(in Chinese). (沙敏, 张丁, 潘仁明, 邢萍, 姜标, 化学学报, 2015, 73, 395.)
(h) Gou, B.; Yang, C.; Zhang, L.; Xia, W. Acta Chim. Sinica 2017, 75, 66(in Chinese). (苟宝权, 杨超, 张磊, 夏吾炯, 化学学报, 2017, 75, 66.)
(i) Zhang, P.; Lu, L.; Shen, Q. Acta Chim. Sinica 2017, 75, 744(in Chinese). (张盼盼, 吕龙, 沈其龙, 化学学报, 2017, 75, 744.)
(j) Wang, J.; Li, F.; Xu, Y.; Wang, J.; Wu, Z.; Yang, C.; Liu, L. Chin. J. Org. Chem. 2018, 38, 1155.
(k) Wang, Q.; Gao, K.; Zou, J.; Zeng, R. Chin. J. Org. Chem. 2018, 38, 863(in Chinese). (王清, 高克成, 邹建平, 曾润生, 有机化学, 2018, 38, 863.)
(l) Wang, D.; Yuan, Z.; Liu, Q.; Chen, P.; Liu, G. Chin. J. Chem. 2018, 36, 507.
[2] (a) Hu, M.; He, Z.; Gao, B.; Li, L.; Ni, C.; Hu, J. J. Am. Chem. Soc. 2013, 135, 17302.
(b) Müller, K.; Faeh, C.; Diederich, F. Science 2007, 317, 1881.
(c) Zhou, N.; Xu, P.; Li, W.; Cheng, Y.; Zhu, C. Acta Chim. Sinica 2017, 75, 60(in Chinese). (周能能, 胥攀, 李伟鹏, 成义祥, 朱成建, 化学学报, 2017, 75, 60.)
(d) Rong, J.; Ni, C.; Wang, Y.; Kuang, C.; Gu, Y.; Hu, J. Acta Chim. Sinica 2017, 75, 105(in Chinese). (荣健, 倪传法, 王云泽, 匡翠文, 顾玉诚, 胡金波, 化学学报, 2017, 75, 105.)
(e) Sun, X.; Wang, W.; Ma, J.; Yu, S. Acta Chim. Sinica 2017, 75, 115(in Chinese). (孙晓阳, 王文敏, 马晶, 俞寿云, 化学学报, 2017, 75, 115.)
(f) Xu, J.; Chen, P.; Ye, J.; Liu, G. Acta Chim. Sinica 2015, 73, 1294(in Chinese). (徐佳斌, 陈品红, 叶金星, 刘国生, 化学学报, 2015, 73, 1294.)
(g) Zhao, X.; Li, T.; Tian, M.; Su, Z.; Wei, A.; Lu, K. Chin. J. Org. Chem. 2018, 38, 677.
(h) Liu, L.; Huang, D.; Wang, Y.; Wen, L.; Yang, Z.; Su, Y.; Wang, K.; Hu, Y. Chin. J. Org. Chem. 2018, 38, 1469.
(i) Liu, Q.; Hu, X. Chin. J. Org. Chem. 2018, 38, 1525.
(j) Gu, Y.; Lu, C.; Gu, Y.; Shen, Q. Chin. J. Chem. 2018, 36, 55.
(k) Fu, X.-P.; Xiao, Y.-L.; Zhang, X. Chin. J. Chem. 2018, 36, 143.
(l) Shi, H.; Lai, B.; Chen, S.; Zhou, X.; Nie, J.; Ma, J.-A. Chin. J. Chem. 2017, 35, 1693.
[3] (a) Okoromoba, O. E.; Han, J.; Hammond, G. B.; Xu, B. J. Am. Chem. Soc. 2014, 136, 14381.
(b) Dutheuil, G.; Couve-Bonnaire, S.; Pannecoucke, X. Angew. Chem., Int. Ed. 2007, 46, 1290.
(c) Liu, T.-L.; Wu, J. E.; Zhao, Y. Chem. Sci. 2017, 8, 3885.
(d) Jakobsche, C. E.; Peris, G.; Miller, S. J. Angew. Chem., Int. Ed. 2008, 47, 6707.
(e) Sommer, H.; Fürstner, A. Chem. Eur. J. 2017, 23, 558.
[4] (a) Daubresse, N.; Chupeau, Y.; Francesch, C.; Lapierre, C.; Pollet, B.; Rolando, C. Chem. Commun. 1997, 1489.
(b) Chen, C. Y.-C. J. Taiwan Inst. Chem. Eng. 2009, 40, 155.
(c) Haidoune, M. B.; Raynaud, I.; O'Connor, N.; Richomme, P.; Mornet, R.; Laloue, M. J. Agric. Food Chem. 1998, 46, 1577.
(d) Lin, J.; Toscano, P. J.; Welch, J. T. Proc. Natl. Acad. Sci. U. S. A. 1998, 95, 14020.
(e) Van der Veken, P.; Senten, K.; Kertèsz, I.; De Meester, I.; Lambeir, A.-M.; Maes, M.-B.; Scharpé, S.; Haemers, A.; Augustyns, K. J. Med. Chem. 2005, 48, 1768.
(f) Liu, Q.; Shen, X.; Ni, C.; Hu, J. Angew. Chem., Int. Ed. 2017, 56, 619.
(g) Lu, X.; He, S.-J.; Cheng, W.-M.; Shi, J. Chin. Chem. Lett. 2018, 29, 1001.
[5] (a) Zhang, X.; Cao, S. Tetrahedron Lett. 2017, 58, 375.
(b) Novikov, M. A.; Nefedov, O. M. Org. Biomol. Chem. 2018, 16, 4963.
(c) Yokota, M.; Fujita, D.; Ichikawa, J. Org. Lett. 2007, 9, 4639.
(d) Takachi, M.; Kita, Y.; Tobisu, M.; Fukumoto, Y.; Chatani, N. Angew. Chem., Int. Ed. 2010, 49, 8717.
(e) Xu, L.; Zhang, Q.; Xie, Q.; Huang, B.; Dai, J.-J.; Xu, J.; Xu, H.-J. Chem. Commun. 2018, 54, 4406.
(f) Zhao, Y.; Jiang, F.; Hu, J. J. Am. Chem. Soc. 2015, 137, 5199.
(g) Kojima, R.; Kubota, K.; Ito, H. Chem. Commun. 2017, 53, 10688.
[6] (a) Zhang, X.; Lin, Y.; Zhang, J.; Cao, S. RSC Adv. 2015, 5, 7905.
(b) Zell, D.; Dhawa, U.; Müller, V.; Bursch, M.; Grimme, S.; Ackermann, L. ACS Catal. 2017, 7, 4209.
(c) Sakaguchi, H.; Uetake, Y.; Ohashi, M.; Niwa, T.; Ogoshi, S.; Hosoya, T. J. Am. Chem. Soc. 2017, 139, 12855.
(d) Tan, D.-H.; Lin, E.; Ji, W.-W.; Zeng, Y.-F.; Fan, W.-X.; Li, Q.; Gao, H.; Wang, H. Adv. Synth. Catal. 2018, 360, 1032.
(e) Hayashi, S.-i.; Nakai, T.; Ishikawa, N. Chem. Lett. 1980, 9, 935.
(f) Zhang, B.; Zhang, X.; Hao, J.; Yang, C. Org. Lett. 2017, 19, 1780.
(g) Fuchibe, K.; Mayumi, Y.; Zhao, N.; Watanabe, S.; Yokota, M.; Ichikawa, J. Angew. Chem., Int. Ed. 2013, 52, 7825.
(h) Ichitsuka, T.; Fujita, T.; Arita, T.; Ichikawa, J. Angew. Chem., Int. Ed. 2014, 53, 7564.
(i) Sakaguchi, H.; Ohashi, M.; Ogoshi, S. Angew. Chem., Int. Ed. 2018, 57, 328.
(j) Cong, Z.-S.; Li, Y.-G.; Chen, L.; Xing, F.; Du, G.-F.; Gu, C.-Z.; He, L. Org. Biomol. Chem. 2017, 15, 3863.
(k) Xiong, Y.; Huang, T.; Ji, X.; Wu, J.; Cao, S. Org. Biomol. Chem. 2015, 13, 7389.
(l) Dai, W.; Shi, H.; Zhao, X.; Cao, S. Org. Lett. 2016, 18, 4284.
(m) Yang, L.; Ji, W.-W.; Lin, E.; Li, J.-L.; Fan, W.-X.; Li, Q.; Wang, H. Org. Lett. 2018, 20, 1924.
(n) Li, J.; Lefebvre, Q.; Yang, H.; Zhao, Y.; Fu, H. Chem. Commun. 2017, 53, 10299.
(o) Xing, B.; Ni, C.; Hu, J. Chin. J. Chem. 2018, 36, 206.
(p) Zhang, Z.; Zhou, Q.; Yu, W.; Li, T.; Zhang, Y.; Wang, J. Chin. J. Chem. 2017, 35, 387.
[7] Tian, P.; Feng, C.; Loh, T.-P. Nat. Commun. 2015, 6, 7472.
[8] (a) Kong, L.; Zhou, X.; Li, X. Org. Lett. 2016, 18, 6320.
(b) Wu, J.-Q.; Zhang, S.-S.; Gao, H.; Qi, Z.; Zhou, C.-J.; Ji, W.-W.; Liu, Y.; Chen, Y.; Li, Q.; Li, X.; Wang, H. J. Am. Chem. Soc. 2017, 139, 3537.
(c) Ji, W.-W.; Lin, E.; Li, Q.; Wang, H. Chem. Commun. 2017, 53, 5665.
[9] Xie, J.; Yu, J.; Rudolph, M.; Rominger, F.; Hashmi, A. S. K. Angew. Chem., Int. Ed. 2016, 55, 9416.
[10] Thornbury, R. T.; Toste, F. D. Angew. Chem., Int. Ed. 2016, 55, 11629.
[11] Zhang, J.; Dai, W.; Liu, Q.; Cao, S. Org. Lett. 2017, 19, 3283.
[12] Hu, J.; Han, X.; Yuan, Y.; Shi, Z. Angew. Chem., Int. Ed. 2017, 56, 13342.
[13] (a) Lu, X.; Wang, Y.; Zhang, B.; Pi, J.-J.; Wang, X.-X.; Gong, T.-J.; Xiao, B.; Fu, Y. J. Am. Chem. Soc. 2017, 139, 12632.
(b) Xu, J.; Ahmed, E.-A.; Xiao, B.; Lu, Q.-Q.; Wang, Y.-L.; Yu, C.-G.; Fu, Y. Angew. Chem., Int. Ed. 2015, 54, 8231.
(c) Xu, J.; Fu, Y.; Luo, D.-F.; Jiang, Y.-Y.; Xiao, B.; Liu, Z.-J.; Gong, T.-J.; Liu, L. J. Am. Chem. Soc. 2011, 133, 15300.
[14] (a) Lu, X.; Xiao, B.; Zhang, Z.; Gong, T.; Su, W.; Yi, J.; Fu, Y.; Liu, L. Nat. Commun. 2016, 7, 11129.
(b) Lu, X. Ph.D. Dissertation, University of Science and Technology of China, Hefei, 2016(in Chinese). (陆熹, 博士论文, 中国科学技术大学, 合肥, 2016.)
(c) Xiao, Y.; Pan, Q.; Zhang, X. Acta Chim. Sinica 2015, 73, 383(in Chinese). (肖玉兰, 潘强, 张新刚, 化学学报, 2015, 73, 383.)
(d) Xu, J.; Xiao, B.; Xie, C.-Q.; Luo, D.-F.; Liu, L.; Fu, Y. Angew. Chem., Int. Ed. 2012, 51, 12551.
[15] Lu, X.; Xiao, B.; Liu, L.; Fu, Y. Chem. Eur. J. 2016, 22, 11161.
[16] Yi, J.; Lu, X.; Sun, Y.-Y.; Xiao, B.; Liu, L. Angew. Chem., Int. Ed. 2013, 52, 12409.
[17] (a) González-Bobes, F.; Fu, G. C. J. Am. Chem. Soc. 2006, 128, 5360.
(b) Zultanski, S. L.; Fu, G. C. J. Am. Chem. Soc. 2013, 135, 624.
(c) Zhou, J.; Fu, G. C. J. Am. Chem. Soc. 2004, 126, 1340.
/
| 〈 |
|
〉 |