

A Novel Yellow Thermally Activated Delayed Fluorescence Emitter For Highly Efficient Organic Light-Emitting Diodes
Received date: 2018-10-18
Online published: 2018-12-20
Supported by
Project supported by the National Natural Science Foundation of China (No. 51773029) and Henan Natural Science Foundation (No. 182300410230).
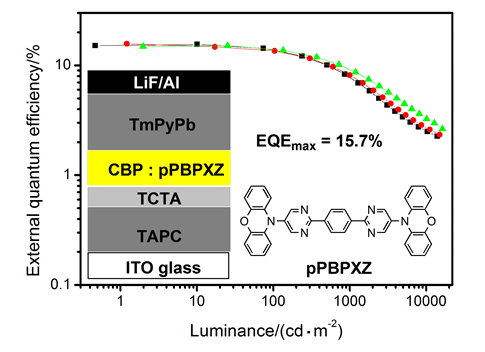
A novel yellow thermally activated delayed fluorescence emitter pPBPXZ was successfully synthesized using phenoxazine as electron-donor and pyrimidine as electron-acceptor by Buchwald-Hartwig and Suzuki coupling reactions. Density functional theory calculations show that pPBPXZ has highly twisted structure with the dihedrals of nearly 90° between phenoxazine and pyrimidine units, while the dihedrals between benzene ring and adjacent pyrimidine rings are almost 0°. The highest occupied molecular orbital (HOMO) is mainly confined on two phenoxazine segments, the lowest unoccupied molecular orbital (LUMO) is mainly located on the central pyrimidine and benzene segments, and there is only a slight overlap between HOMO and LUMO. Cyclic voltammetry investigation show pPBPXZ has reversible redox process, and the HOMO and LUMO energy levels were estimated to be -5.43 eV and -3.23 eV, respectively, from the onsets of oxidation and reduction curves. In diluted toluene solution, pPBPXZ exhibits the absorption band assigned to intramolecular charge-transfer transition and yellow fluorescence with a structureless emission peak at 535 nm. From the onsets of the fluorescence and phosphorescence spectra of pPBPXZ in 2Me-THF at 77 K, the lowest singlet (S1) and the lowest triplet (T1) energy levels are calculated to be 2.57 eV and 2.48 eV, respectively, and thus △EST is only 0.09 eV. The doped electroluminescence devices using pPBPXZ as guest emitter were prepared by vacuum evaporation method. These devices with doping ratios (w) of 6%, 11%, 16% and 23% show yellow emission at 552~560 nm and low turn-on voltages of 3.1~3.3 V. The device with a doping ratio of 11% exhibits the highest maximum forward-viewing efficiencies of a maximum current efficiency of 49.9 cd/A, a maximum power efficiency of 49.0 lm/W and a maximum external quantum efficiency of 15.7% without any light out-coupling enhancement. Particularly, the efficiencies of these devices are not sensitive to the doping ratios of pPBPXZ, which would benefit the further practical application.
Wang Zhiqiang , Cai Jialin , Zhang Ming , Zheng Caijun , Ji Baoming . A Novel Yellow Thermally Activated Delayed Fluorescence Emitter For Highly Efficient Organic Light-Emitting Diodes[J]. Acta Chimica Sinica, 2019 , 77(3) : 263 -268 . DOI: 10.6023/A18100437
[1] Sun, Y. R.; Giebink, N. C.; Kanno, H.; Ma, B. W.; Thompson, M. E.; Forrest, S. R. Nature 2006, 440, 908.
[2] Reineke, S.; Lindner, F.; Schwartz, G.; Seidler, N.; Walzer, K.; Lussem, B.; Leo, K. Nature 2009, 459, 234.
[3] Helander, M. G.; Wang, Z. B.; Qiu, J.; Greiner, M. T.; Puzzo, D. P.; Liu, Z. W. Science 2011, 332, 944.
[4] Han, T.-H.; Lee, Y.; Choi, M.-R.; Woo, S.-H.; Hong, B. H.; Ahn, J.-H.; Lee, T.-W. Nat. Photonics 2012, 459, 105.
[5] Sasabe, H.; Kido, J. J. Mater. Chem. C 2013, 1, 1699.
[6] Zhang, Z.; Li, W.; Ye, K.; Zhang, H. Acta Chim. Sinica 2016, 74, 179(in Chinese). (张振宇, 李婉君, 叶开其, 张红雨, 化学学报, 2016, 74, 179.)
[7] Yu, Y.; Yang, J.; Ren, Z.; Xie, G.; Li, Q.; Li, Z. Acta Chim. Sinica 2016, 74, 865.
[8] Wang, F.; Cao, X.; Mei, L.; Zhang, X.; Hu, J.; Tao, Y. Chinese J. Chem. 2018, 36, 241.
[9] Liang, X.; Wang, Z.; Wang, L.; Hanif, M.; Hu, D.; Su, S.; Xie, Z.; Gao, Y.; Yang, B.; Ma, Y. Chinese J. Chem. 2017, 35, 1559.
[10] Lin, D.; Song, S.; Chen, Z.; Guo, P.; Chen, J.; Shi, H.; Mai, Y.; Song, H. Chinese J. Org. Chem. 2018, 38, 103(in Chinese). (林丹燕, 宋森川, 陈智勇, 郭鹏然, 陈江韩, 史华红, 麦裕良, 宋化灿, 有机化学, 2018, 38, 103.)
[11] Xu, H.; Chen, R.; Sun, Q.; Huang, W.; Liu, X. Chem. Soc. Rev. 2014, 43, 3259.
[12] Volz, D.; Wallesch, M.; Fléchon, C.; Danz, M.; Verma, A.; Navarro, J. M.; Zink, D. M.; Bräse, S.; Baumann, T. Green Chem. 2015, 17, 1988.
[13] Chi, Y.; Tong, B.; Chou, P.-T. Coord. Chem. Rev. 2014, 281, 1.
[14] Uoyama, H.; Goushi, K.; Shizu, K.; Nomura, H.; Adachi, C. Nature 2012, 492, 234.
[15] Tao, Y.; Yuan, K.; Chen, T.; Xu, P.; Li, H.; Chen, R.; Zheng, C.; Zhang, L.; Huang, W. Adv. Mater. 2014, 26, 7931.
[16] Kim, M.; Jeon, S. K.; Hwang, S. H.; Lee, S. S.; Yu, E.; Lee, J. Y. Chem. Commun. 2016, 52, 339.
[17] Lee, J.; Aizawa, N.; Yasuda, T. Chem. Mater. 2017, 29, 8012.
[18] Tamai, Y.; Ohkita, H.; Benten, H.; Ito, S.; Lee, J.-H.; Kido, J.; Park, J. J. Mater. Chem. C 2013, 1, 432.
[19] Chen, W.-C.; Lee, C.-S.; Tong, Q.-X. J. Mater. Chem. C 2015, 3, 10957.
[20] Chen, Y.-H.; Lin, C.-C.; Huang, M.-J.; Hung, K.; Wu, Y.-C.; Lin, W.-C.; Chen-Cheng, R.-W.; Lin, H.-W.; Cheng, C.-H. Chem. Sci. 2016, 7, 4044.
[21] Pan, Y.; Li, W.; Zhang, S.; Yao, L.; Gu, C.; Xu, H.; Yang, B.; Ma, Y. Adv. Opt. Mater. 2014, 2, 510.
[22] Konidena, R. K.; Thomas, K. R. J.; Dubey, D. K.; Sahoo, S.; Jou, J.-H. Chem. Commun. 2017, 53, 11802.
[23] Chen, W.-C.; Yuan, Y.; Ni, S.-F.; Tong, Q.-X.; Wong, F.-L.; Lee, C.-S. Chem. Sci. 2017, 8, 3599.
[24] Wang, Z.; Li, Y.; Cai, X.; Chen, D.; Xie, G.; Liu, K.; Wu, Y.; Lo, C.-C.; Lien, A.; Cao, Y.; Su, S.-J. ACS Appl. Mater. Interfaces 2016, 8, 8627.
[25] Chen, D.-Y.; Liu, W.; Zheng, C.-J.; Wang, K.; Li, F.; Tao, S.-L.; Ou, X.-M.; Zhang, X.-H. ACS Appl. Mater. Interfaces 2016, 8, 16791.
[26] Lee, D. R.; Choi, J. M.; Lee, C. W.; Lee, J. Y. ACS Appl. Mater. Interfaces 2016, 8, 23190.
[27] Cai, X.; Li, X.; Xie, G.; He, Z.; Gao, K.; Liu, K.; Chen, D.; Cao, Y.; Su, S.-J. Chem. Sci. 2016, 7, 4264.
[28] Li, F.; Xie, G.; Gong, S.; Wu, K.; Yang, C. Chem. Sci. 2016, 7, 5441.
[29] Rajamalli, P.; Senthilkumar, N.; Gandeepan, P.; Huang, P.-Y.; Huang, M.-J.; Yang, C.-Y.; Chiu, M.-J.; Chu, L.-K.; Lin, H.-W.; Cheng, C.-H. J. Am. Chem. Soc. 2016, 138, 628.
[30] Liu, X.; Zhan, C.; Zheng, C.; Liu, C.; Lee, C.; Li, F.; Ou, X.; Zhang, X. Adv. Mater. 2015, 27, 2378.
[31] Wang, K.; Zheng, C. J.; Liu, W.; Liang, K.; Shi, Y. Z.; Tao, S. L.; Lee, C. S.; Ou, X. M.; Zhang, X. H. Adv. Mater. 2017, 29, 1701476.
[32] Yang, Z.; Mao, Z.; Xie, Z.; Zhang, Y.; Liu, S.; Zhao, J.; Xu, J.; Chi, Z.; Aldred, M. P. Soc. Rev. 2017, 46, 915.
[33] Liu, Y.; Li, C.; Ren, Z.; Yan, S.; Bryce, M. R. Nat. Rev. Mater. 2018, 3, 18020.
[34] Wong, M. Y.; Zysman-Colman, E. Adv. Mater. 2017, 29, 1605444.
[35] Xiang, Y.; Gong, S.; Zhao, Y.; Yin, X.; Luo, J.; Wu, K.; Lu, Z.; Yang, C. J. Mater. Chem. C 2016, 4, 9998.
[36] Xie, G.; Li, X.; Chen, D.; Wang, Z.; Cai, X.; Chen, D.; Li, Y.; Liu, K.; Cao, Y.; Su, S. J. Adv. Mater. 2016, 28, 181.
[37] Chen, J.-X.; Wang, K.; Zheng, C.-J.; Zhang, M.; Shi, Y.-Z.; Tao, S.-L.; Lin, H.; Liu, W.; Tao, W.-W.; Ou, X.-M.; Zhang, X.-H. Adv. Sci. 2018, 5, 1800436.
[38] Chen, X.; Yang, Z.; Xie, Z.; Zhao, J.; Yang, Z.; Aldred, M. P.; Chi, Z. Mater. Chem. Front. 2018, 2, 1017.
[39] Chen, Y.; Li, S.; Hu, T.; Wei, X.; Li, Z.; Liu, W.; Liu, J.; Wang, R.; Yi, Y.; Zhao, C.; Wang, Y.; Wang, P. J. Mater. Chem. C 2018, 6, 2951.
[40] Xiang, Y.; Zhu, Z.-L.; Xie, D.; Gong, S.; Wu, K.; Xie, G.; Lee, C.-S.; Yang, C. J. Mater. Chem. C 2018, 6, 7111.
[41] Huang, J.; Nie, H.; Zeng, J.; Zhuang, Z.; Gan, S.; Cai, Y.; Guo, J.; Su, S.-J. Angew. Chem. Int. Ed. 2017, 56, 12971.
[42] Li, J.; Ding, D. X.; Tao, Y. T.; Wei, Y. Y.; Chen, R. F.; Xie, L. H.; Haung, W.; Xu, H. Adv. Mater. 2016, 28, 3122.
[43] Pan, K.-C.; Li, S.-W.; Ho, Y.-Y.; Shiu, Y.-J.; Tsai, W.-L.; Jiao, M.; Lee, W.-K.; Wu, C.-C.; Chung, C.-L.; Chatterjee, T.; Li, Y.-S.; Wong, K.-T.; Hu, H.-C.; Chen, C.-C.; Lee, M.-T. Adv. Funct. Mater. 2016, 26, 7560.
/
| 〈 |
|
〉 |