

Study on the Mechanism of Frustrated Lewis Pairs Catalysed Hydrogenation of 2,3-Disubstituted 2H-1,4-Benzoxazine
Received date: 2018-11-13
Online published: 2019-01-31
Supported by
Project supported by the National Natural Science Foundation of China (Nos. 21705029, 21701131).
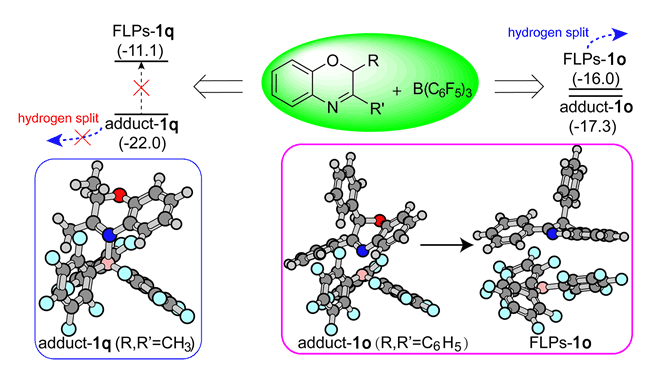
Due to the different reactivity of hydrogenation reaction by metal-free FLPs catalyst for 2,3-disubstituted 2H-1,4-benzoxazine, we explored the reaction mechanism by density functional theory calculations. We have chosen three kinds of substrates with different hydrogenation reactivity as the prototype substrates and toluene as the solvent to calculate the potential energy profile for the FLPs-catalysed hydrogenation reaction at M06-2X/6-311++G(d,p) level with polarized continuum model (PCM) to simulate the solvent effect. From the potential energy profile, we found that when B(C6F5)3 encounters with 2,3-diphenyl 2H-1,4-benzoxazine (1o) or 2-methyl-3-phenyl 2H-1,4-benzoxazine (1p) in toluene, it mainly generates the mixture of Lewis acid-base adducts and Frustrated Lewis Pairs, which has almost similar stability suggesting the transformation of each other by intermolecular rearrangement. However, it reveals big difference when the B(C6F5)3 encounters with 2,3-dimethyl 2H-1,4-benzoxazine (1q), where the Lewis acid-base adducts is the preference rather than the mixture of Lewis acid-base adducts and Frustrated Lewis Pairs or Frustrated Lewis Pairs since the lower stability energy. Due to the big energy gap (10.9 kcal/mol) between Lewis acid-base adducts and Frustrated Lewis Pairs, the generated Lewis acid-base adducts could not transform into Frustrated Lewis Pairs in the FLPs-catalysed hydrogenation of 1q at 298 K. That is the main reason why 1q is an inert substrate for the hydrogenation catalysed by FLPs. Natural Bond Orbital, Mulliken charge analysis and the proton affinity energy of N4 site was carried out to assess the electric effect of substituent at C3 on N4 site. It reveals negligible effect of substituent at C3 on N4 charge (basicity) and thus proposes that steric hindrance effect is the major factor affecting the stability energy of Lewis acid-base adducts and Frustrated Lewis Pairs. This is confirmed further by calculative investigation about the substituent effect (-CH2CH3, -CH(CH3)2 and C(CH3)3) on the stability of Lewis acid-base adducts and Frustrated Lewis Pairs in 2-methyl-3-substituted 2H-1,4-benzoxazine, where with the increased steric hindrance effect, Lewis acid-base adducts tend to have similar stability with Frustrated Lewis Pairs even though less stability. These results clearly illustrate the elusive phenomenon in our previous experiment and may provide new insight for the design of another novel FLPs-catalysed hydrogenation reaction.
Wei Simin , Wang Yinghui , Zhao Hongmei . Study on the Mechanism of Frustrated Lewis Pairs Catalysed Hydrogenation of 2,3-Disubstituted 2H-1,4-Benzoxazine[J]. Acta Chimica Sinica, 2019 , 77(3) : 278 -286 . DOI: 10.6023/A18110461
[1] Hey, D. A.; Reich, R. M.; Baratta, W.; Kuhn, F. E. Coord. Chem. Rev. 2018, 374, 114.
[2] Lux, S.; Baldauf-Sommerbauer, G.; Siebenhofer, M. ChemSusChem 2018, 11, 3357.
[3] Liu, W. P.; Sahoo, B.; Junge, K.; Beller, M. Acc. Chem. Res. 2018, 51, 1858.
[4] Ye, R. P.; Lin, L.; Li, Q. H.; Zhou, Z. F.; Wang, T. T.; Russell, C. K.; Adidharma, H.; Xu, Z. H.; Yao, Y. G.; Fan, M. H. Catal. Sci. Technol. 2018, 8, 3428.
[5] Song, J. J.; Huang, Z. F.; Pan, L.; Li, K.; Zhang, X. W.; Wang, L.; Zou, J. J. Appl. Catal. B-Environ. 2018, 227, 386.
[6] Rayhan, U.; Kowser, Z.; Islam, M. N.; Redshaw, C.; Yamato, T. Top. Catal. 2018, 61, 560.
[7] Orlandi, M.; Brenna, D.; Harms, R.; Jost, S.; Benaglia, M. Org. Process Res. Dev. 2018, 22, 430.
[8] Filonenko, G. A.; van Putten, R.; Hensen, E. J. M.; Pidko, E. A. Chem. Soc. Rev. 2018, 47, 1459.
[9] Meemken, F.; Baiker, A. Chem. Rev. 2017, 117, 11522.
[10] Schauermann, S. J. Phys. Chem. Lett. 2018, 9, 5555.
[11] Meemken, F.; Rodriguez-Garcia, L. J. Phys. Chem. Lett. 2018, 9, 996.
[12] Xie, J. H.; Zhou, Q. L. Acta Chim. Sinica 2012, 70, 1427. (谢建华, 周其林, 化学学报, 2012, 70, 1427.)
[13] Yamaguchi, R.; Ikeda, C.; Takahashi, Y.; Fujita, K.-i. J. Am. Chem. Soc. 2009, 131, 8410.
[14] Monfette, S.; Turner, Z. R.; Semproni, S. P.; Chirik, P. J. J. Am. Chem. Soc. 2012, 134, 4561.
[15] Hu, S. B.; Chen, M. W.; Zhai, X. Y.; Zhou, Y. G. Acta Chim. Sinica 2018, 76, 103. (胡书博, 陈木旺, 翟小勇, 周永贵, 化学学报, 2018, 76, 103.)
[16] Zhang, Q.; Liu, A.; Yu, H. Z.; Fu, Y. Acta Chim. Sinica 2018, 76, 113. (张琪, 刘奥, 于海珠, 傅尧, 化学学报, 2018, 76, 113.)
[17] Liu, X.; Han, Z. B.; Wang, Z.; Ding, K. L. Acta Chim. Sinica 2014, 72, 849. (刘旭, 韩召斌, 王正, 丁奎岭, 化学学报, 2014, 72, 849.)
[18] Jiang, W.; Zhao, Q.; Tang, W. Chin. J. Chem. 2018, 36, 153.
[19] Xia, J. Z.; Nie, Y.; Yang, G. Q.; Liu, Y. G.; Gridnev, I. D.; Zhang, W. B. Chin. J. Chem. 2018, 36, 612.
[20] Zhang, Y. W.; Chen, Y. L.; Fang, X. L.; Yuan, Y. Z.; Zhu, H. P. Chin. J. Org. Chem. 2017, 37, 2275. (张亦伟, 陈艺林, 方霄龙, 袁友珠, 朱红平, 有机化学, 2017, 37, 2275.)
[21] Welch, G. C.; Juan, R. R. S.; Masuda, J. D.; Stephan, D. W. Science 2006, 314, 1124.
[22] Stephan, D. W. Acc. Chem. Res. 2015, 48, 306.
[23] Liu, Y. B.; Du, H. F. Acta Chim. Sinica 2014, 72, 771. (刘勇兵, 杜海峰, 化学学报, 2014, 72, 771.)
[24] Meng, W.; Feng, X. Q.; Du, H. F. Acc. Chem. Res. 2018, 51, 191.
[25] Wang, H.; Zheng, Y.; Pan, Z. T.; Fu, H. L.; Ling, F.; Zhong, W. H. Chin. J. Org. Chem. 2017, 37, 301. (王辉, 郑亿, 潘振涛, 傅鸿樑, 凌飞, 钟为慧, 有机化学, 2017, 37, 301.)
[26] Mömming, C. M.; Frömel, S.; Kehr, G.; Fröhlich, R.; Grimme, S.; Erker, G. J. Am. Chem. Soc. 2009, 131, 12280.
[27] Mahdi, T.; Heiden, Z. M.; Grimme, S.; Stephan, D. W. J. Am. Chem. Soc. 2012, 134, 4088.
[28] Zhang, Z.; Du, H. Angew. Chem. Int. Ed. 2015, 54, 623.
[29] Liu, Y. B.; Du, H. F. J. Am. Chem. Soc. 2013, 135, 6810.
[30] Liu, Y. B.; Du, H. F. J. Am. Chem. Soc. 2013, 135, 12968.
[31] Wei, S. M.; Du, H. F. J. Am. Chem. Soc. 2014, 136, 12261.
[32] Ren, X. Y.; Du, H. F. J. Am. Chem. Soc. 2016, 138, 810.
[33] Fasano, V.; Curless, L. D.; Radcliffe, J. E.; Ingleson, M. J. Angew. Chem.-Int. Ed. 2017, 56, 9202.
[34] Mahdi, T.; Stephan, D. W. J. Am. Chem. Soc. 2014, 136, 15809.
[35] Scott, D. J.; Fuchter, M. J.; Ashley, A. E. J. Am. Chem. Soc. 2014, 136, 15813.
[36] Brown, K. S.; Djerassi, C. J. Am. Chem. Soc. 1964, 86, 2451.
[37] McAllister, S. D.; Rizvi, G.; Anavi-Goffer, S.; Hurst, D. P.; Barnett-Norris, J.; Lynch, D. L.; Reggio, P. H.; Abood, M. E. J. Med. Chem. 2003, 46, 5139.
[38] Wang, A. H.; Prouty, C. P.; Pelton, P. D.; Yong, M.; Demarest, K. T.; Murray, W. V.; Kuo, G. H. Bioorg. Med. Chem. Lett. 2010, 20, 1432.
[39] Shim, J. Y.; Collantes, E. R.; Welsh, W. J.; Subramaniam, B.; Howlett, A. C.; Eissenstat, M. A.; Ward, S. J. J. Med. Chem. 1998, 41, 4521.
[40] Wei, S. M.; Feng, X. Q.; Du, H. F. Org. Biomol. Chem. 2016, 14, 8026.
[41] Zhao, Y.; Truhlar, D. G. Theor. Chem. Acc. 2008, 120, 215.
[42] Wang, Y. H.; Jie, J. L.; Zhao, H. M.; Bai, Y.; Qin, P. X.; Song, D. Acta Chim. Sinica 2018, 76, 475. (王英辉, 节家龙, 赵红梅, 白羽, 秦佩萱, 宋迪, 化学学报, 2018, 76, 475.)
[43] Huang, F.; Jiang, J. L.; Wen, M. W.; Wang, Z. X. J. Theor. Comput. Chem. 2014, 13, 1350074.
[44] Zhao, J. Y.; Wang, G. Q.; Li, S. H. Dalton Trans. 2015, 44, 9200.
[45] Rokob, T. A.; Hamza, A.; Papai, I. J. Am. Chem. Soc. 2009, 131, 10701.
[46] Antinolo, A.; Carrillo-Hermosilla, F.; Fernandez-Galan, R.; Martinez-Ferrer, J.; Alonso-Moreno, C.; Bravo, I.; Moreno-Blazquez, S.; Salgado, M.; Villasenor, E.; Albaladejo, J. Dalton Trans. 2016, 45, 10717.
[47] Zhao, L.; Li, H.; Lu, G.; Huang, F.; Zhang, C.; Wang, Z.-X. Dalton Trans. 2011, 40, 1929.
[48] Rokob, T. A.; Hamza, A.; Stirling, A.; Pápai, I. J. Am. Chem. Soc. 2009, 131, 2029.
[49] Das, S.; Pati, S. K. Chem.-Eur. J. 2017, 23, 1078.
[50] Lu, Z. P.; Cheng, Z. H.; Chen, Z. X.; Weng, L. H.; Li, Z. H.; Wang, H. D. Angew. Chem.-Int. Ed. 2011, 50, 12227.
[51] Gao, S. L.; Wu, W.; Mo, Y. R. Int. J. Quantum Chem. 2011, 111, 3761.
[52] Frisch, M. J.; Trucks, G. W.; Schlegel, H. B.; Scuseria, G. E.; Robb, M. A.; Cheeseman, J. R.; Montgomery, J. A.; Vreven, T.; Kudin, K. N.; Burant, J. C.; Millam, J. M.; Iyengar, S. S.; Tomasi, J.; Barone, V.; Mennucci, B.; Cossi, M.; Scalmani, G.; Rega, N.; Petersson, G. A.; Nakatsuji, H.; Hada, M.; Ehara, M.; Toyota, K.; Fukuda, R.; Ha-segawa, J.; Ishida, M.; Nakajima, T.; Honda, Y.; Kitao, O.; Nakai, H.; Klene, M.; Li, X.; Knox, J. E.; Hratchian, H. P.; Cross, J. B.; Bakken, V.; Adamo, C.; Jaramillo, J.; Gomperts, R.; Stratmann, R. E.; Yazyev, O.; Austin, A. J.; Cammi, R.; Pomelli, C.; Ochterski, J. W.; Ayala, P. Y.; Morokuma, K.; Voth, G. A.; Salvador, P.; Dannen-berg, J. J.; Zakrzewski, V. G.; Dapprich, S.; Daniels, A. D.; Strain, M. C.; Farkas, O.; Malick, D. K.; Rabuck, A. D.; Raghavachari, K.; Foresman, J. B.; Ortiz, J. V.; Cui, Q.; Baboul, A. G.; Clifford, S.; Cioslowski, J.; Stefanov, B. B.; Liu, G.; Liashenko, A.; Piskorz, P.; Komaromi, I.; Martin, R. L.; Fox, D. J.; Keith, T.; Al-Laham, M. A.; Peng, C. Y.; Nanayakkara, A.; Challacombe, M.; Gill, P. M. W.; Johnson, B.; Chen, W.; Wong, M. W.; Gonzalez, C.; Pople, J. A. Gaussian 09, Revision A. 01, Gaussian, Inc, Wallingford, CT, 2009.
/
| 〈 |
|
〉 |