

Phosphoric Acid Based Porous Aromatic Framework for Uranium Extraction
Received date: 2019-01-16
Online published: 2019-02-14
Supported by
Project supported by the National Basic Research Program of China (973 Program, No. 2014CB931804) and the National Natural Science Foundation of China (NSFC Project, Nos. 91622106, 21531003, 21601031).
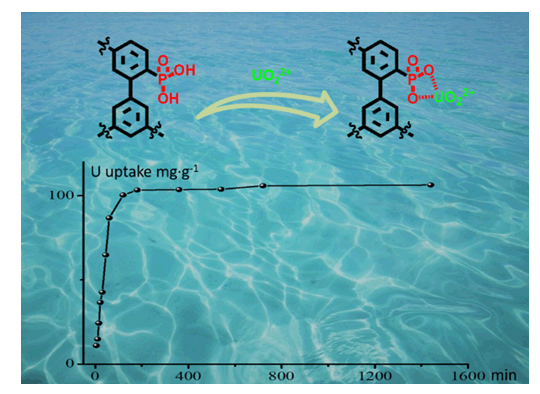
As a clean, safe, efficient, and economical energy, nuclear energy plays an irreplaceable role in the resource sector. However, uranium deposits on land will run out in the coming decades. The uranium content in seawater is huge but its concentration is as low as~3 ppb. So it is an urgent problem to design and synthesize adsorbent materials with high extraction efficiency. In this paper, taking the actual industrialization as the direction, we adopted biphenyl as the building block and synthesize the porous aromatic framework material (PAF-45) in a low price. Then porous aromatic framework material (PAF-45-PG) with phosphoric acid groups was prepared through a post-modification procedure. The structure and pore characteristics of the compound were investigated by FTIR, TGA, PXRD, SEM, TEM and N2 adsorption experiments. FTIR spectrum indicates that the emerging vibrational peaks at 900~1250 cm-1 can be ascribed to the successful decoration of phosphate groups in PAF-45-PG compared with pure PAF-45. Powder X-ray diffraction shows that PAF-45 and PAF-45-PG are amorphous. And transmission electron microscope (TEM) images also agree with the conclusion of PXRD that PAF materials possess disordered structure. Moreover, there is no significant weight loss before 400℃ demonstrated by thermogravimetric analysis, which indicates the high thermal stability of two PAF resultants. The porosity of PAF networks was characterized by measuring the N2 adsorption isotherm at 77 K. Calculated by Brunauer-Emmett-Teller (BET) adsorption model, the specific surface area of PAF-45-PG is 426 m2·g-1, which is lower than that of pure PAF-45 (828 m2·g-1). This reduction of surface area is attributed to the introduction of functional groups which increase the weight per constitutional unit and occupy the space in the porous structure. After that, we tested the UO22+ ion adsorption of PAFs in simulated seawater. The equilibrium adsorption capacity of PAF-45-PG increases with the increase of uranium concentration, and reaches the maximum value (101 mg·g-1) at about 8 ppm. Because the maximum capacity of PAF-45 is 5.9 mg·g-1, this result indicates that the adsorption of uranium ion in PAF-45-PG is mainly caused by the post-modified phosphate functional group on its pore surface. Due to the low cost and simple preparation process, the material (PAF-45-PG) has a great industrial prospect.
Li Zhangnan , Sha Haoyan , Yang Nan , Yuan Ye , Zhu Guangshan . Phosphoric Acid Based Porous Aromatic Framework for Uranium Extraction[J]. Acta Chimica Sinica, 2019 , 77(5) : 469 -474 . DOI: 10.6023/A19010028
[1] Kim, J.; Tsouris, C.; Mayes, R. T.; Oyola, Y.; Saito, T.; Janke, C. J.; Dai, S.; Schneider, E.; Sachde, D. Sep. Sci. Technol. 2013, 48, 367.
[2] Yue, Y.; Mayes, R. T.; Gill, G.; Kuo, L.; Wood, J.; Binder, A.; Brown, S.; Dai, S. RSC Adv. 2015, 5, 50005.
[3] Kim, J.; Tsouris, C.; Oyola, Y.; Janke, C. J.; Mayes, R. T.; Dai, S.; Gill, G.; Kuo, L.; Wood, J.; Choe, K.; Schneider, E.; Lindner, H. Ind. Eng. Chem. Res. 2014, 53, 6076.
[4] Saito, T.; Brown, S.; Chatterjee, S.; Kim, J.; Tsouris, C.; Mayes, R.T.; Kuo, L.; Gill, G.; Oyola, Y.; Janke, C. J.; Dai, S. J. Mater. Chem. A 2014, 2, 14674.
[5] Kim, J.; Oyola, Y.; Tsouris, C.; Cole, C. R.; Mayes, R. T.; Janke, J. C.; Dai, S. Ind. Eng. Chem. Res. 2013, 52, 9433.
[6] Sholl, D. S.; Lively, R. P. Nature 2016, 532, 435.
[7] Lu, Y. Nat. Chem. 2014, 6, 175.
[8] Yue, Y.; Mayes, R.; Kim, J.; Sun, X.; Chen, J.; Dai, S. Angew. Chem., Int. Ed. 2013, 52, 13458.
[9] Liu, C.; Xie, J.; Zhao, J.; Wu, T.; Wang, H.; Liu, W.; Zhang, J.; Cui, Y. Nat. Energy. 2017, 2, 17007.
[10] Feng, M.; Sarma, D.; Qi, X.; Du, K.; Huang, X. J. Am. Chem. Soc. 2016, 138, 12578.
[11] Sun, Q.; Aguila, B. Adv. Mater. 2018, 1705479.
[12] Barber, P. S.; Kelley, S. P.; Griggs, C. S.; Wallace, S.; Rogers, R. D. Green Chem. 2014, 16, 1828.
[13] Yue, Y.; Sun, X.; Mayes, R. T.; Kim, J.; Fulvio, P. F.; Qiao, Z.; Brown, S.; Tsouris, C.; Oyola, Y.; Dai, S. Sci. China: Chem. 2013, 56, 1510.
[14] Kobuke, Y.; Tabushi, I.; Aoki, T.; Kamaishi, T.; Hagiwara, I. Ind. Eng. Chem. Res. 1988, 27, 1461.
[15] Carboni, M.; Abney, C. W.; Liu, S.; Lin, W. Chem. Sci. 2013, 4, 2396.
[16] Manos, M. J.; Kanatzidis, M. G. J. Am. Chem. Soc. 2012, 134, 16441.
[17] Kobayashi, S.; Tokunoh, M.; Saegusa, T.; Mashio, F. Macromolecules 1985, 18, 2357.
[18] Chen, H. J.; Huang, S. Y.; Zhang, Z. B.; Liu, Y. H.; Wang, X. K. Acta Chim. Sinica 2017, 75, 560(in Chinese). (陈海军, 黄舒怡, 张志宾, 刘云海, 王祥科, 化学学报, 2017, 75, 560.)
[19] Chatterjee, S.; Bryantsev, V. S.; Brown, S.; Johnson, J. C.; Grant, C. D.; Mayes, R. T.; Hay, B. P. Ind. Eng. Chem. Res. 2016, 55, 4161.
[20] Yue, Y.; Zhang, C.; Tang, Q.; Mayes, R. T.; Liao, W.; Liao, C.; Dai, S. Ind. Eng. Chem. Res. 2016, 55, 4125.
[21] Shao, D.; Wang, X.; Ren, X.; Hu, S.; Wen, J.; Tan, Z.; Marwani, H. M. J. Ind. Eng. Chem. 2018, 67, 380.
[22] Birnbaum, J. C.; Busche, B.; Lin, Y.; Shaw, W. J.; Fryxell, G. E. Chem. Commun. 2002, 1374.
[23] Ren, X.; Yang, S.; Tan, X.; Chen, C.; Sheng, G.; Wang, X. J. Hazard. Mater. 2012, 237, 199.
[24] Yantasee, W.; Fryxell, G. E.; Addleman, R. S.; Wiacek, R. J.; Koonsiripaiboon, V.; Pattamakomsan, K.; Xu, J.; Raymond, K. N. J. Hazard. Mater. 2009, 168, 1233.
[25] Ma, T.; Yuan, Z. Dalton Trans. 2010, 39, 9570.
[26] Venkateswarlu, S.; Yoon, M. RSC Adv. 2015, 5, 65444.
[27] Zhu, Y.; Liu, Y.; Ren, T.; Yuan, Z. Nanoscale 2014, 6, 6627.
[28] Das, S.; Pandey, A. K.; Athawale, A. A.; Natarajan, V.; Manchanda, V. K. Water Treat. 2012, 38, 1140.
[29] Yang, Y.; Yan, Z.; Wang, L.; Meng, Q.; Yuan, Y.; Zhu, G. J. Mater. Chem. A 2018, 6, 5202.
[30] Yuan, Y.; Sun, F.; Zhang, F.; Ren, H.; Jing, X.; Gao, X. Adv. Mater. 2013, 25, 6619.
[31] Yuan, Y.; Sun, F.; Li, L.; Cui, P.; Zhu, G. Nat. Commun. 2014, 4260.
[32] Li, L.-N. Ph.D. Dissertation, Jilin University, Changchun, 2015 (in Chinese). (李莉娜, 博士论文, 吉林大学, 长春, 2015.)
[33] Egawa, H.; Nonaka, T.; Ikari, M. J. Appl. Polym. Sci. 2010, 29, 2045.
/
| 〈 |
|
〉 |