

Synthesis of β,γ-Unsaturated Esters and γ-Ketone Esters with Amino Acid Ester-Derived Katritzky Salts
Received date: 2019-04-08
Online published: 2019-05-22
Supported by
Project supported by the Natural Science Foundation of the Jiangsu Higher Education Institutions of China(18KJA350001)
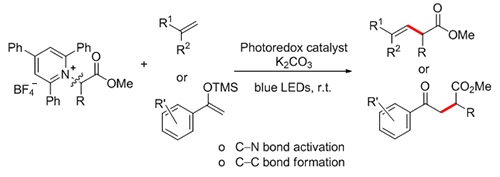
β,γ-Unsaturated ester and γ-ketone ester are important synthons, which can be used to convert into various heterocyclic compounds, natural products and pharmaceuticals. The development of efficient methods for the synthesis of β,γ-unsaturated ester and γ-ketone ester compounds has attracted much attention from synthetic chemists. By using Katritzky pyridinium salts as radical precursors, commercially available Ru(bpy)3Cl2?6H2O as photocatalyst, K2CO3 as base, and dichloromethane (DCM) as solvent, we developed a simple and efficient method for the synthesis of a series of β,γ-unsaturated esters and γ-ketone esters by C—N bond activation. Bench-stable and easily handled redox-active Katritzky pyridinium salts derived from abundant amino acids were used as radical precursors for the alkylation of 1,1-diarylethylene and aryl enol silyl ether species upon irradiation with household blue LEDs. The reaction displays an excellent functional group tolerance and a potential utility for amino acids functionalization, allowing to access desired products in moderate to good yields. Moreover, under air conditions, the reaction has moderate compatibility. Scaling up the reaction in grams, the yield was higher and the target product was obtained with 91% yield. Control experiments demonstrated that the photocatalyst and visible light were both essential for the success of the reaction. Performing the reaction in the presence of radical scavenger TEMPO, did lead to no desired product 3a formation. Moreover, a TEMPO-trapped product was determined by MS analysis and NMR, indicating a radical-type mechanism of this reaction. It is of note that this protocol could offer a powerful complementary strategy for the use of amino acids that were also employed in photoredox-catalyzed decarboxylative reactions. A representative procedure for this reaction is as following: A 10 mL oven-dried Schlenk-tube was charged with 1a (111.5 mg, 0.20 mmol), Ru(bpy)3Cl2?6H2O (3.0 mg, 2 mol%), K2CO3 (55.2 mg, 0.40 mmol) and a magnetic stirring bar. The tube was evacuated and back-filled three times with argon. A solution of 2a (53 μL, 0.30 mmol) in DCM (2 mL) was injected into the tube by syringe. The resulting mixture was stirred at room temperature upon irradiation with blue LEDs (22 W) and monitored by thin-layer chromatography (TLC). After completion, the solvent was then removed under reduced pressure and the residue was purified by flash column chromatography on silica gel to give 3a as an off-white solid (42.7 mg, 65% yield).
Yong Zhao, , Shihong Li, , Miaomiao Zhang, , Feng Liu, . Synthesis of β,γ-Unsaturated Esters and γ-Ketone Esters with Amino Acid Ester-Derived Katritzky Salts[J]. Acta Chimica Sinica, 2019 , 77(9) : 916 -921 . DOI: 10.6023/A19040121
| [1] | For selected reviews see: (a) Zhang, J.-R.; Xu, L.; Liao, Y.-Y.; Deng, J.-C.; Tang, R.-Y . Chin. J. Che. 2017, 35, 271; |
| [1] | (b) Yin, X.; Li, W.; Zhao, B.; Cheng, K . Chin. J. Org. Che. 2018, 38, 2879. |
| [1] | ( 殷晓婷, 李文炅, 赵保丽, 程凯 , 有机化学. 2018, 38, 2879.) |
| [2] | For selected reviews, see: (a) Jin, Y.; Fu, H . Asian J. Org. Chem. 2017, 6, 368; |
| [2] | (b) Skubi, K. L.; Blum, T. R.; Yoon, T. P . Chem. Rev. 2016, 116, 10035; |
| [2] | (c) Xuan, J.; Zhang, Z.-G.; Xiao, W.-J . Angew. Chem., Int. Ed. 2015, 54, 15632; |
| [2] | (d) Wang, D.; Zhang, L.; Luo, S . Acta Chim. Sinica. 2017, 75, 22. |
| [2] | ( 王德红, 张龙, 罗三中 , 化学学报, 2017, 75, 22.) |
| [3] | For selected recent examples, see.(a) Kautzky, J. A.; Wang, T.; Evans, R. W.; MacMillan, D. W. C . J. Am. Chem. So. 2018, 140, 6522; |
| [3] | (b) Bloom, S.; Liu, C.; K?lmel, D. K.; Qiao, J.-X.; Zhang, Y.; Poss, M. A.; Ewing, W. R.; MacMillan, D. W. C . Nature Chem. 2018, 10, 205; |
| [3] | (c) Zhao, Y.; Chen, J.-R.; Xiao, W.-J . Org. Lett. 2018, 20, 224; |
| [3] | (d) Cheng, W.-M.; Shang, R.; Fu, M.-C.; Fu, Y . Chem. Eur. J. 2017, 23, 2537; |
| [3] | (e) Wang, D.; Zhu, N.; Chen, P.; Lin, Z.; Liu, G . J. Am. Chem. Soc. 2017, 139, 15632; |
| [3] | (f) Fawcett, A.; Pradeilles, J.; Wang, Y.; Mutsuga, T.; Myers, E. L.; Aggarwal, V. K . Science. 2017, 357, 283; |
| [3] | (g) Garza-Sanchez, R. A.; Tlahuext-Aca, A.; Tavakoli, G.; Glorius, F . ACS Catal. 2017, 7, 4057; |
| [3] | (h) Cheng, W.-M.; Shang, R.; Fu, Y . ACS Catal. 2017, 7, 907; |
| [3] | (i) McCarver, S. J.; Qiao, J.-X.; Carpenter, J.; Borzilleri, R. M.; Poss, M. A.; Eastgate, M. D.; Miller, M.; MacMillan, D. W. C . Angew. Chem., Int. Ed. 2017, 56, 728; |
| [3] | (j) Johnston, C. P.; Smith, R.; Allmendinger, T. S.; MacMillan, D. W. C . Nature. 2016, 536, 322; |
| [3] | (k) Müller, D. S.; Untiedt, N. L.; Dieskau, A. P.; Lackner, G. L.; Overman, L. E . J. Am. Chem. Soc. 2015, 137, 660. |
| [4] | For selected recent examples, see: (a) Zhou, W.-J.; Cao, G.-M.; Shen, G.; Zhu, X.-Y.; Gui, Y.-Y.; Ye, J.-H.; Sun, L.; Liao, L.-L.; Li, J.; Yu, D.-G . Angew. Chem., Int. Ed. 2017, 5, 15683; |
| [4] | (b) Nuhant, P.; Oderinde, M. S.; Genovino, J.; Juneau, A.; Gagné, Y.; Allais, C.; Chinigo, G. M.; Choi, C.; Sach, N. W.; Bernier, L.; Fobian, Y. M.; Bundesmann, M. W.; Khunte, B.; Frenette, M.; Fadeyi, O. O . Angew. Chem., Int. Ed. 2017, 56, 15309; |
| [4] | (c) Zhang, P.; Le, C.; MacMillan, D. W. C . J. Am. Chem. Soc. 2016, 138, 8084; |
| [4] | (d) Feng, Z.; Min, Q.-Q.; Zhao, H.-Y.; Gu, J.-W.; Zhang, X . Angew. Chem., Int. Ed. 2015, 54, 1270; |
| [4] | (e) Iqbal, N.; Choi, S.; Kim, E.; Cho, E. J . J. Org. Chem. 2012, 77, 11383. |
| [5] | For selected recent examples, see.(a) Lima, F.; Sharma, U. K.; Grunenberg, L.; Saha, D.; Johannsen, S.; Sedelmeier, J.; Van der Eycken, E. V Ley, S. V . Angew. Chem., Int. Ed. 2017, 56, 15136; |
| [5] | (b) Matsui, J. K.; Primer, D. N.; Molander, G. A . Chem. Sci. 2017, 8, 3512; |
| [5] | (c) Amani, J.; Molander, G. A . Org. Lett. 2017, 19, 3612; |
| [5] | (d) Primer, D. N.; Molander, G. A . J. Am. Chem. Soc. 2017, 139, 9847; |
| [5] | (e) Lima, F.; Kabeshov, M. A.; Tran, D. N.; Battilocchio, C.; Sedelmeier, J.; Sedelmeier, G.; Schenkel, B.; Ley, S. V . Angew. Chem., Int. Ed. 2016, 55, 14085; |
| [5] | (f) Huo, H.; Harms, K.; Meggers, E . J. Am. Chem. Soc. 2016, 138, 6936; |
| [5] | (g) El Khatib, M.; Serafim, R. A. M.; Molander, G. A . Angew. Chem., Int. Ed. 2016, 55, 254; |
| [5] | (h) Primer, D. N.; Karakaya, I.; Tellis, J. C.; Molander, G. A . J. Am. Chem. Soc. 2015, 137, 2195. |
| [6] | For selected recent examples, see.(a) Lang, S. B.; Wiles, R. J.; Kelly, C. B.; Molander, G . Angew. Chem., Int. Ed. 2017, 56, 15073; |
| [6] | (b) Zheng, S.; Primer, D. N.; Molander, G. A . ACS Catal. 2017, 7, 7957; |
| [6] | (c) Remeur, C.; Kelly, C. B.; Patel, N. R.; Molander, G. A . ACS Catal. 2017, 7, 6065; |
| [6] | (d) Lin, K.; Wiles, R. J.; Kelly, C. B.; Davies, G. H. M.; Molander, G. A . ACS Catal. 2017, 7, 5129; |
| [6] | (e) Patel, N. R.; Kelly, C. B.; Siegenfeld, A. P.; Molander, G. A . ACS Catal. 2017, 7, 1766; |
| [6] | (f) Deng, Y.; Liu, Q. Smith, A. B . J. Am. Chem. Soc. 2017, 139, 9487; |
| [6] | (g) Jouffroy, M.; Primer, D. N.; Molander, G. A . J. Am. Chem. Soc. 2016, 138, 475; |
| [6] | (h) Corc, V.; Chamoreau, L.-M.; Derat, E.; Goddard, J.-P.; Ollivier, C.; Fen-sterbank, L . Angew. Chem., Int. Ed. 2015, 54, 11414. |
| [7] | For selected recent examples, see.(a) Slutskyy, Y.; Jamison, C. R.; Zhao, P.; Lee, J.; Rhee, Y. H.; Overman, L. E . J. Am. Chem. Soc. 2017, 139, 7192; |
| [7] | (b) Zhang, X.; MacMillan, D W. C. . J. Am. Chem. So. 2016, 138, 13862; |
| [7] | (c) Lackner, G. L.; Quasdorf, K. W.; Pratsch, G.; Overman, L. E . J. Org. Chem. 2015, 80, 6012; |
| [7] | (d) Nawrat, C. C.; Jamison, C. R.; Slutskyy, Y.; MacMillan, D. W. C.; Overman, L. E . J. Am. Chem. Soc. 2015, 137, 11270. |
| [8] | The Generation of Aryl Radicals Can be Achieved via the Reductive Cleavage of C(sp2)—N Bonds of the Aryl Diazonium Salts, For a review, see: Ghosh, I.; Marzo, L.; Das, A.; Shaikh, R.; Ko?nig, B. Acc. Chem. Res. 2016, 49, 1566. |
| [9] | (a) Eds.: Pollegioni, L.; Servi, S . Nonnatural Amino Acids: Methods and Protocols, Springer, New York, 2012, pp. 1~249; |
| [9] | (b) Ager, D. J . Amino Acids, Peptides and Proteins in Organic Chemistry, Ed.: Hughes, A. B., Wiley-VCH, Weinheim, 2009, Vol. 1, pp. 495~526. |
| [10] | Katritzky, A. R.; Gruntz, U.; Kenny, D. H.; Rezende, M. C.; Sheikh, H . J. Chem. Soc. Perkin Trans. 1. 1979,430. |
| [11] | Ouyang, K.; Hao, W.; Zhang, W.-X.; Xi, Z . Chem. Re. 2015, 115, 12045. |
| [12] | (a) Basch, C. H.; Liao, J.; Xu, J.; Piane, J. J.; Watson, M. P . J. Am. Chem. Soc. 2017, 139, 5313; |
| [12] | (b) Liao, J.; Guan, W.; Boscoe, B. P.; Tucker, J. W.; Tomlin, J. W.; Garnsey, M. R.; Watson, M. P . Org. Lett. 2018, 20, 3030; |
| [12] | (c) Guan, W.; Liao, J.; Watson, M. P . Synthesis. 2018, 5. 3231. |
| [13] | Grimshaw, J.; Moore, S.; Grimshaw, J. T . Acta Chem. Scand. Ser. 1983, 37, 485. |
| [14] | (a) Klauck, F. J. R.; James, M. J.; Glorius, F . Angew. Chem., Int. Ed. 2017, 56, 12336; |
| [14] | (b) Klauck, F. J. R.; Yoon, H.; James, M. J.; Lautens, M.; Glorius, F . ACS Catal. 2019, 9, 236; |
| [14] | (c) Sandfort, F.; Strieth- Kalthoff, F.; Klauck, F. J. R.; James, M. J.; Glorius, F . Chem. Eur. J. 2018, 2. 17210. |
| [15] | (a) Wu, J.-J.; He, L.; Noble, A.; Aggarwal, V. K . J. Am. Chem. So. 2018, 140, 10700; |
| [15] | (b) Wu, J.-J.; Grant, P. S.; Li, X.-B.; Noble, A.; Aggarwal, V. K . Angew. Chem., Int. Ed. 2019, 58, 10. 10.1002/anie.201814452. |
| [16] | Hu, J.; Wang, G.; Li, S.; Shi, Z . Angew. Chem., Int. Ed. 2018, 57, 15227. |
| [17] | Ociepa, M.; Turkowska, J.; Gryko, D . ACS Cata. 2018, 8, 11362. |
| [18] | (a) Zhu, Z.-F.; Zhang, M.-M.; Liu, F . Org. Biomol. Chem. 2019, 17, 1531 |
| [18] | (b) Zhang, M.-M.; Liu, F . Org. Chem. Front. 2018, 5, 3443. |
| [19] | Brase, S.; Waegell, B.; de Meijere, A . Synthesi. 1998, 2, 148. |
| [20] | Ikeda, Y.; Nakamura, T.; Yorimitsu, H.; Oshima, K . J. Am. Chem. Soc. 2002, 124, 6514. |
| [21] | Tang, S.; Liu, K.; Liu, C.; Lei, A.-W . Chem. Soc. Rev. 2015, 44, 1070. |
| [22] | For selected recent examples with aliphatic carboxylic acids, see.(a) Cao, H.; Jiang, H.; Feng, H.; Kwan, J. M. C.; Liu, X.; Wu, J . J. Am. Chem. Soc. 2018, 140, 16360; |
| [22] | (b) Zhou, H.; Ge, L.; Song, J.; Jian, W.; Li, Y.; Li, C.; Bao, H . iScience. 2018, 3, 255; |
| [22] | (c) Wang, G.-Z.; Shang, R.; Fu, Y . Org. Lett. 2018, 20, 888; |
| [22] | (d) Koy, M.; Sandfort, F.; Tlahuext-Aca, A.; Quach, L.; Daniliuc, C. G.; Glorius, F . Chem. Eur. J. 2018, 24, 4552; |
| [22] | (e) Zhu, N.; Zhao, J.; Bao, H . Chem. Sci. 2017, 8, 2081. |
| [23] | For selected examples with alkyl halides, see.(a) Xiong, H.; Li, Y.; Qian, B.; Wei, R.; Van der Eycken, E. V.; Bao, H . Org. Lett. 2019, 21, 776; |
| [23] | (b) Kurandina, D.; Rivas, M.; Radzhabov, M.; Gevorgyan, V . Org. Lett. 2018, 20, 357; |
| [23] | (c) Wang, G.-Z.; Shang, R.; Cheng, W.-M.; Fu, Y . J. Am. Chem. Soc. 2017, 139, 18307; |
| [23] | (d) Kurandina, D.; Parasram, M.; Gevorgyan, V . Angew. Chem., Int. Ed. 2017, 56, 14212; |
| [23] | (e) Liu, W.; Li, L.; Chen, Z.; Li, C.-J . Org. Biomol. Chem. 2015, 13, 6170; |
| [23] | (f) Weiss, M. E.; Kreis, L. M.; Lauber, A.; Carreira, E. M . Angew. Chem., Int. Ed. 2011, 50, 11125; |
| [23] | (g) Affo, W. H.; Fujioka, T.; Ikeda, Y.; Nakamura, T.; Yorimitsu, H.; Oshima, K.; Imamura, Y.; Mizuta, T.; Miyoshi, K . J. Am. Chem. Soc. 2006, 128, 8068; |
| [23] | (h) Na, Y. G.; Park, S. Y.; Han, S. B.; Han, H.; Ko, S. W.; Chang, S . J. Am. Chem. Soc. 2004, 126, 250. |
| [24] | (a) Liu, C.; Tang, S.; Liu, D.; Yuan, J.; Zheng, L.; Meng, L.; Lei, A.-W . Angew. Chem., Int. Ed. 2012, 51, 3638; |
| [24] | (b) Nishikata, T.; Noda, Y.; Fujimoto, R.; Sakashita, T . J. Am. Chem. Soc. 2013, 135, 16372; |
| [24] | (c) Liu, Q.; Yi, H.; Liu, J.; Yang, Y.-H.; Zhang, X.; Zeng, Z.-Q.; Lei, A.-W . Chem. Eur. J. 2013, 1. 5120. |
| [25] | Jiang, X.; Zhang, M.-M.; Xiong, W.; Lu, L.-Q.; Xiao, W.-J . Angew. Chem., Int. Ed. 2019, 58, 2402. |
/
| 〈 |
|
〉 |