

Hierarchical In2S3/CdIn2S4 Heterostructured Nanohybrids as Photocatalyst for Coupling of Benzyl Amines under Visible Light
Received date: 2019-04-04
Online published: 2019-06-05
Supported by
Project supported by the National Natural Science Foundation of China (No. 21563026), the Program for Changjiang Scholars and Innovative Research Team in University (No. IRT15R56), and the Innovation Team Basic Scientific Research Project of Gansu Province (No. 1606RJIA324).
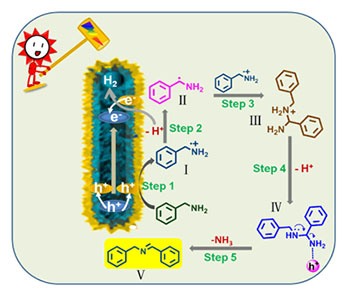
It is a more attractive strategy that the selective oxidation of amines to the corresponding imines driven by visible light and photocatalyst. However, it is still necessary to look further into the mechanism of the transformation. In order to advance a new green photocatalysts system under milder conditions, hierarchical In2S3 nanotubes, which maintained the mother skeleton of NH2-MIL-68(In), was prepared from NH2-MIL-68(In) and thiourea, and a cation exchange method was used to synthesize hierarchical In2S3/CdIn2S4 heterostructured composites. The structure, morphology and photoelectric properties of the catalysts were characterized by X-ray diffraction (XRD), scanning electron microscope (SEM), transmission electron microscopy (TEM), high-resolution transmission electron microscopy (HRTEM), ultraviolet-visible diffuse reflectance spectroscopy (UV-vis DRS), fluorescence spectroscopy (PL) and electrochemical impedance spectra (EIS) analysis. The results showed that the existence of heterojunction between In2S3 and CdIn2S4 could minimize the recombination rate of photogenerated electron-hole pairs, which made the In2S3/CdIn2S4 show superior catalytic activity. The results of the evaluated experiments under visible light illumination showed that lots of synergy existed between the hierarchical structure, and the heterostructure could help to enhance the efficiency of carriers transfer and separation, which resulting in a high photocatalytic efficiency of In2S3/CdIn2S4 nanohybrids for the oxidation coupling of benzyl amines to imines under visible light irradiation. Interestingly, the further investigation concerned to the reaction condition revealed that the product could be detected not only in air but also in N2 conditions, which burst out of the restriction of the reported reaction conditions and made the condition of the conversion become milder. The possible photocatalytic mechanism for the transformation was investigated by a series of experiments which concerned to the catching of the active species. The results showed that the reason for the occurring of the reaction was initiated by the presence of the nitrogen-centered radical cations and the carbon-centered radicals which were induced by the photogenerated hole (h+) in the photocatalyst. As for these situations, the conditions for the conversion of benzyl amines to imines were milder than the reported ones ever. What's more, from our experiments, the catalysts can be recycled five times at least, which indicated good reusability of hierarchical In2S3/CdIn2S4 nanohybrids.
Liu Ruxue , He Xiaoyan , Niu Litong , Lv Bolin , Yu Fei , Zhang Zhe , Yang Zhiwang . Hierarchical In2S3/CdIn2S4 Heterostructured Nanohybrids as Photocatalyst for Coupling of Benzyl Amines under Visible Light[J]. Acta Chimica Sinica, 2019 , 77(7) : 653 -660 . DOI: 10.6023/A19040113
[1] Alvaro, M.; Carbonell, E.; Ferrer, B.; i Xamena, F. X. L.; Garcia, H. Chem. Eur. J. 2007, 13, 5106.
[2] Sun, D.; Li, Z. Chin. J. Chem. 2017, 35, 135.
[3] Chouhan, A.; Pilet, G.; Daniele, S.; Pandey, A. Chin. J. Chem. 2017, 35, 209.
[4] Zhang, W.; Li, Q.; Yang, X.; Ma, Z.; Wang, H.; Wang, X. Acta Chim. Sinica 2017, 75, 80. (张文强, 李秋艳, 杨馨雨, 马征, 王欢欢, 王晓军, 化学学报, 2017, 75, 80.)
[5] Shen, K.; Chen, X.; Chen, J.; Li, Y. ACS Catal. 2016, 6, 5887.
[6] Jiao, L.; Wang, Y.; Jiang, H. L.; Xu, Q. Adv. Mater. 2018, 30, 1703663.
[7] Fang, Y.; Zhu, S. R.; Wu, M. K.; Zhao, W. N.; Han, L. J. Solid State Chem. 2018, 266, 205.
[8] Feng, J.; Yang, Z.; He, S.; Niu, X.; Zhang, T.; Ding, A.; Liang, H.; Feng, X. Chemosphere 2018, 212, 114.
[9] Li, X.; Weng, B.; Zhang, N.; Xu, Y. J. RSC Adv. 2014, 4, 64484.
[10] Yan, B.; Zhang, L.; Tang, Z.; Al-Mamun, M.; Zhao, H.; Su, X. Appl. Catal. B:Environ. 2017, 218, 743.
[11] Xu, X.; Liu, R.; Cui, Y.; Liang, X.; Lei, C.; Meng, S.; Ma, Y.; Lei, Z.; Yang, Z. Appl. Catal. B:Environ. 2017, 210, 484.
[12] Liu, L.; Ding, L.; Liu, Y.; An, W.; Lin, S.; Liang, Y.; Cui, W. Appl. Catal. B:Environ. 2017, 201, 92.
[13] Peng, S.; Li, L.; Wu, Y.; Jia, L.; Tian, L. L.; Srinivasan, M.; Ramakrishna, S.; Yan, Q.; Mhaisalkar, S. G. CrystEngComm 2013, 15, 1922.
[14] Yu, B. J.; Su, Y.; Cheng, B. Adv. Funct. Mater. 2007, 17, 1984.
[15] Li, X.; Yu, J.; Jaroniec, M. Chem. Soc. Rev. 2016, 45, 2603.
[16] Shen, R.; Jiang, C.; Xiang, Q.; Xie, J.; Li, X. Appl. Surf. Sci. 2019, 471, 43.
[17] Mao, C.; Cheng, H.; Tian, H.; Li, H.; Xiao, W. J.; Xu, H.; Zhao, J.; Zhang, L. Appl. Catal. B:Environ. 2018, 228, 87.
[18] Meng, S.; Ye, X.; Ning, X.; Xie, M.; Fu, X.; Chen, S. Appl. Catal. B:Environ. 2016, 182, 356.
[19] Yang, Z.; Xu, X.; Liang, X.; Lei, C.; Gao, L.; Hao, R.; Lu, D.; Lei, Z. Appl. Surf. Sci. 2017, 420, 276.
[20] Lang, X.; Chen, X.; Zhao, J. Chem. Soc. Rev. 2014, 43, 473.
[21] Xu, Y.; Chen, Y.; Fu, W. F. Appl. Catal. B:Environ. 2018, 236, 176.
[22] Proctor, A. D.; Panuganti, S.; Bartlett, B. M. Chem. Commun. 2018, 54, 1101.
[23] Raza, F.; Park, J. H.; Lee, H. R.; Kim, H. I.; Jeon, S. J.; Kim, J. H. ACS Catal. 2016, 6, 2754.
[24] Sun, D.; Ye, L.; Li, Z. Appl. Catal. B:Environ. 2015, 164, 428.
[25] Liu, H.; Xu, C.; Li, D.; Jiang, H. Angew. Chem. 2018, 130, 5477.
[26] Wang, S.; Guan, B. Y.; Lu, Y.; Lou, X. J. Am. Chem. Soc. 2017, 139, 17305.
[27] Zhang, Y.; Zhou, J.; Chen, X.; Feng, Q.; Cai, W. J. Alloys Compd. 2019, 777, 109.
[28] Samanta, S.; Khilari, S.; Srivastava, R. ACS Appl. Nano Mater. 2018, 1, 426.
[29] Wang, M.; Li, L.; Lu, J.; Luo, N.; Zhang, X.; Wang, F. Green Chem. 2017, 19, 5172.
[30] Liang, R.; Shen, L.; Jing, F.; Wu, W.; Qin, N.; Lin, R.; Wu, L. Appl. Catal. B:Environ. 2015, 162, 245.
[31] Peng, Y.; Zhao, M.; Chen, B.; Zhang, Z.; Huang, Y.; Dai, F.; Lai, Z.; Cui, X.; Tan, C.; Zhang, H. Adv. Mater. 2018, 30, 1705454.
[32] Zhao, K.; Zhang, X.; Zhang, L. Electrochem. Commun. 2009, 11, 612.
/
| 〈 |
|
〉 |