

4-Substituted Hantzsch Esters as Alkylation Reagents in Organic Synthesis
Received date: 2019-05-12
Online published: 2019-06-12
Supported by
Project supported by the National Natural Science Foundation of China(Nos.21672037);Project supported by the National Natural Science Foundation of China(21532001)
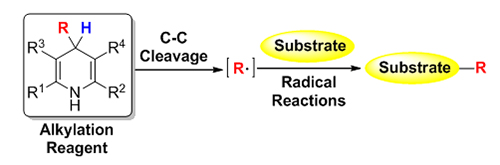
Hantzsch Esters were first synthesized by Arthur Rudolf Hantzsch in 1881, and widely used in pharmaceutical chemistry. The application of Hantsch Esters in organic synthesis in the early time was mainly focused on the dehydrogenation of 1,4-dihydrogen pyridines (DHPs) in the synthesis of functional pyridines. In 1955, Mauzerall and Westheimer found that Malachite Green could be reduced by Hantzsch Esters to generate the hydrogenated product. Then these DHPs were extensively used as a reductant for decades due to their electron and hydrogen donating properties. In recent years, scientist found that C—C bond cleavage at 4-position of 4-substituted Hantzsch Esters would lead alkyl transfer, and the alkylation process was a radical process. With the rapid development of free radical chemistry, various alkylation reactions using 4-substituted Hantzsch Esters as alkylation reagent have been developed, such as addition reactions of imines and alkenes; cross-coupling reactions with aryl halides; substitution reactions with functional aromatics; Tsuji-Trost reaction; radical insertion with sulfur dioxide; and asymmetric alkylation etc. The advantages in alkylation transfer by using 4-substituted Hantzsch Esters as alkyl source in the past five years were witnessed dramatically: (1) Highly toxic alkyl metal reagents could be avoided in the alkylation reactions; (2) Compared with the moisture sensitivity of alkyl metal reagents Hantzsch Esters are easily handling; (3) 1,4-Dihydrogen pyridines (DHPs) are biologically-inspired model molecular of reduced nicotinamide adenine dinucleotide (NADH), which would expand the application in biosynthesis. A brief summary in this field is presented in this review, and the advances are classified according to different reaction types. Although these creativity works were developed, there are still some challenges: (1) Could aromatic groups at 4-position of 4-substituted Hantzsch Esters serve as arylation reagents? (2) How to recover the rest pyridine part of Hantzsch Esters after alkylation; (3) New type reactions need to be developed for the asymmetric synthesis.
Shengqing Ye, , Jie Wu, . 4-Substituted Hantzsch Esters as Alkylation Reagents in Organic Synthesis[J]. Acta Chimica Sinica, 2019 , 77(9) : 814 -831 . DOI: 10.6023/A19050170
| [1] | Hantzsch, A . Ber. Dtsch. Chem. Ges. 1881, 14, 1637. |
| [2] | (a) Janis, R. A.; Triggle, D. J. J. Med. Chem. 1983, 25, 775. |
| [2] | (b) Bocker, R. H.; Guengerich, F. P. J. Med. Chem. 1986, 29, 1596. |
| [2] | (c) Xie, W.; Wu, Y.; Zhang, J.; Mei, Q.; Zhang, Y.; Zhu, N.; Liu, R.; Zhang, H. Eur. J. Med. Chem. 2018, 145, 35. |
| [2] | (d) Xie, W.; Zhang, H.; He, J.; Zhang, J.; Yu, Q.; Luo, C.; Li, S. Bioorg. Med. Chem. Lett. 2017, 27, 530. |
| [3] | Bergstrom, F. W. Chem. Rev. 1944, 35, 77. |
| [4] | Mauzerall, D.; Westheimer, F. H. J. Am. Chem. Soc. 1955, 77, 2261. |
| [5] | For selected reviews see: (a) Ouellet, S. G.; Walji, A. M.; Macmillan, D. W. C. Acc. Chem. Res. 2007, 40, 1327. |
| [5] | (b) de Vries, J. G.; Mrsic, N. Catal. Sci. Technol. 2011, 1, 727. |
| [5] | (c) Zheng, C.; You, S.-L. Chem. Soc. Rev. 2012, 41, 2498. |
| [5] | (d) Huang, W.; Cheng, X.. Synlett 2017, 28, 148. |
| [5] | (e) Li, X.; Meng, Y.; Yi, P.; Stepień, M.; Chmielewski, P. J. Angew. Chem., Int. Ed. 2017, 56, 10810. |
| [6] | Loev, B.; Snader, K. M . J. Org. Chem., 1965, 30 1914. |
| [7] | Wei, Z.; Li, J.; Wang, Z.; Li, P.; Wang, Y . Chin. J. Org. Chem. 2017, 37, 1835 (in Chinese). |
| [7] | ( 魏振中, 李江飞, 王泽云, 李品华, 王永秋 , 有机化学, 2017, 37, 1835.) |
| [8] | For selected examples see:(a) Zou, Y.-Q.; H?rmann, F. M.; Bach, T. Chem. Soc. Rev. 2018, 47, 278. |
| [8] | (b) Wang, F.; Chen, P.; Liu, G. Acc. Chem. Res. 2018, 51, 2036. |
| [8] | (c) Wang, K.; Kong, W. Chin. J. Chem. 2018, 36, 247. |
| [8] | (d) Qiu, S.; Wang, C.; Xie, S.; Huang, X.; Chen, L.; Zhao, Y.; Zeng, Z. Chem. Commun. 2018, 54, 11383. |
| [8] | (e) Xie, L.-Y.; Peng, S.; Liu, F.; Chen, G.-R.; Xia, W.; Yu, X.; Li, W.-F.; Cao, Z.; He, W.-M. Org. Chem. Front. 2018, 5, 2604. |
| [8] | (f) Lu, L.-H.; Zhou, S.-J.; He, W.-B.; Xia, W.; Chen, P.; Yu, X.; Xu, X.; He, W.-M. Org. Biomol. Chem. 2018, 16, 9064. |
| [8] | (g) Zheng, Y.; Liu, M.; Qiu, G.; Xie, W.; Wu, J. Tetrahedron 2019, 75, 1663. |
| [8] | (h) Liu, K.-J.; Jiang, S.; Lu, L.-H.; Tang, L.-L.; Tang, S.-S.; Tang, H.-S.; Tang, Z.; He, W.-M.; Xu, X. Green Chem. 2018, 20, 3038. |
| [8] | (i) Xie, L.-Y.; Peng, S.; Liu, F.; Yi, J.-Y.; Wang, M.; Tang, Z.; Xu, X.; He, W.-M. Adv. Synth. Catal. 2018, 360, 4259. |
| [8] | (j) Xie, L.-Y.; Peng, S.; Liu, F.; Chen, G.-R.; Xia, W.; Yu, X.; Li, W.-F.; Cao, Z.; He, W.-M. Org. Chem. Front. 2018, 5, 2604. |
| [8] | (k) Guo, T.; Wei, X.-N.; Liu, Y.; Zhang, P.-K.; Zhao, Y.-H. Org. Chem. Front. 2019, 6, 1414. 1414. |
| [9] | For selected examples see: (a) Yoon, T. P.; Ischay, M. A.; Du, J . Nat. Chem. 2010, 2, 527. |
| [9] | (b) Teply, F. Collect. Czech. Chem. Commun. 2011, 76, 859. |
| [9] | (c) Narayanam, J. M.; Stephenson, C. R. Chem. Soc. Rev. 2011, 40, 102. |
| [9] | (d) Xuan, J.; Xiao, W. J. Angew. Chem., Int. Ed. 2012, 51, 6828. |
| [9] | (e) Shi, L.; Xia, W. Chem. Soc. Rev. 2012, 41, 7687. |
| [9] | (f) Prier, C. K.; Rankic, D. A.; MacMillan, D. W. Chem. Rev. 2013, 113, 5322. |
| [9] | (g) Xi, Y.; Yi, H.; Lei, A. Org. Biomol. Chem. 2013, 11, 2387. |
| [9] | (h) Xuan, J.; Lu, L. Q.; Chen, J. R.; Xiao, W. J. Eur. J. Org. Chem. 2013, 6755. |
| [9] | (i) Hari, D. P.; K?nig, B. Angew. Chem., Int. Ed. 2013, 52, 4734. |
| [9] | (j) Hopkinson, M. N.; Sahoo, B.; Li, J. L.; Glorius, F. Chem. Eur. J. 2014, 20, 3874. |
| [9] | (k) Pe?-López, M.; Rosas-Hernández, A.; Beller, M. Angew. Chem., Int. Ed. 2015, 54, 5006. |
| [9] | (l) Shaw, M. H.; Twilton, J.; MacMillan, D. W. C. J. Org. Chem. 2016, 81, 6898. |
| [9] | (m) Wang, D.; Zhang, L.; Luo, S. Acta Chim. Sinica 2017, 75, 22 (in Chinese) |
| [9] | (王德红, 张龙, 罗三中, 化学学报, 2017, 75, 22.) |
| [10] | Li, G.; Chen, R.; Wu, L.; Fu, Q.; Zhang, X.; Tang, Z. Angew. Chem., Int. Ed. 2013, 52, 8432. |
| [11] | Zhang, H.-H.; Yu, S . J. Org. Chem. 2017, 82, 9995. |
| [12] | Gu, F.; Huang, W.; Liu, X.; Chen, W.; Cheng, X. Adv. Synth. Catal. 2017, 360, 925. |
| [13] | Wu, Q.-Y.; Min, Q.-Q.; Ao, G.-Z.; Liu, F. Org. Biomol. Chem. 2018, 16, 6391. |
| [14] | Mcdonald, B. R.; Scheidt, K. A . Org. Lett. 2018, 20, 6881. |
| [15] | Van Leeuwen, T.; Buzzetti, L.; Perego, L. A.; Melchiorre, P. Angew. Chem., Int. Ed. 2019, 58, 4953. |
| [16] | Milligan, J. A.; Phelan, J. P.; Polites, V. C.; Kelly, C. B.; Molander, G. A . Org. Lett. 2018, 20, 6840. |
| [17] | Chen, H.; Anand, D.; Zhou, L . Asian J. Org. Chem. 2019, 8, 661. |
| [18] | Chen, W.; Liu, Z.; Tian, J.; Li, J.; Ma, J.; Cheng, X.; Li, G. J. Am. Chem. Soc. 2016, 138, 12312. |
| [19] | Nakajima, K.; Nojima, S.; Nishibayashi, Y. Angew. Chem., Int. Ed. 2016, 55, 14106. |
| [20] | Gutiérrez-Bonet, á.; Tellis, J. C.; Matsui, J. K.; Vara, B. A.; Molander, G. A. ACS Catal. 2016, 6, 8004. |
| [21] | Dumoulin, A.; Matsui, J. K.; Gutiérrez-Bonet, á.; Molander, G. A. Angew. Chem., Int. Ed. 2018, 57, 6614. |
| [22] | Badir, S. O.; Dumoulin, A.; Matsui, J. K.; Molander, G. A. Angew. Chem., Int. Ed. 2018, 57, 6610. |
| [23] | Nakajima, K.; Guo, X.; Nishibayashi, Y . Chem. Asian J. 2018, 13, 3653. |
| [24] | Buzzetti, L.; Prieto, A.; Roy, S. R.; Melchiorre, P. Angew. Chem., Int. Ed. 2017, 56, 15039. |
| [25] | For selected examples see: (a) Wu, C.; Lu, L.-H.; Peng, A.-Z.; Jia, G.-K.; Peng, C.; Cao, Z.; Tang, Z.; He, W.-M.; Xu, X . Green Chem. 2018, 20, 3683. |
| [25] | (b) Lu, L.-H.; Zhou, S.-J.; Sun, M.; Chen, J.-L.; Xia, W.; Yu, X.; Xu, X.; He, W.-M. ACS Sustainable Chem. Eng. 2019, 7, 1574. |
| [25] | (c) Wu, C.; Xiao, H.-J.; Wang, S.-W.; Tang, M.-S.; Tang, Z.-L.; Xia, W.; Li, W.-F.; Zhong, C.; He, W.-M. ACS Sustainable Chem. Eng.. 2019, 7, 2169. |
| [25] | (d) Wu, C.; Wang, Z.; Hu, Z.; Zeng, F.; Zhang, X.-Y.; Cao, Z.; Tang, Z.; He, W.-M.; Xu, X. Org. Biomol. Chem.. 2018, 16, 3177. |
| [25] | (e) Wang, Z.; Yang, L.; Liu, H.-L.; Tan, Y.-Z.; Bao, W.-H.; Wang, M.; Tang, Z.; He, W.-M. Chin. J. Org. Chem. 2018, 38, 2639 (in Chinese) |
| [25] | (王峥, 杨柳, 刘慧兰, 谭英芝, 包文虎, 汪明, 唐子龙, 何卫民, 有机化学, 2018, 38, 2639.) |
| [26] | Liu, X.; Liu, R.; Dai, J.; Cheng, X.; Li, G . Org. Lett. 2018, 20, 6906. |
| [27] | Song, Z.-Y.; Zhang, C.-L.; Ye, S. Org. Biomol. Chem. 2019, 17, 181. |
| [28] | Li, G.; Wu, L.; Lv, G.; Liu, H.; Fu, Q.; Zhang, X.; Tang, Z . Chem. Commun. 2014, 50, 6246. |
| [29] | Nakajima, K.; Nojima, S.; Sakata, K.; Nishibayashi, Y . ChemCatChem 2016, 8, 1028. |
| [30] | Wang, Z.-J.; Zheng, S.; Matsui, J. K.; Liu, Z.; Molander, G. A. Chem. Sci. 2019, 10, 4389. |
| [31] | Cao, L.; Zheng, L.; Huang, Q. J. Organomet. Chem. 2014, 768, 56. |
| [32] | For selected examples see: (a) Xie, L.-Y.; Peng, S.; Tan, J.-X.; Sun, R.-X.; Yu, X.; Dai, N.-N.; Tang, Z.-L.; Xu, X.; He, W.-M. ACS Sustainable Chem. Eng. 2018, 6, 16976. |
| [32] | (b) Xie, L.-Y.; Peng, S.; Lu, L.-H.; Hu, J.; Bao, W.-H.; Zeng, F.; Tang, Z.; Xu, X.; He, W.-M. ACS Sustainable Chem. Eng. 2018, 6, 7989. |
| [32] | (c) Xie, L.-Y.; Peng, S.; Jiang, L.-L.; Peng, X.; Xia, W.; Yu, X.; Wang, X.-X.; Cao, Z.; He, W.-M. Org. Chem. Front. 2019, 6, 167. |
| [33] | Gutiérrez-Bonet, á.; Remeur, C.; Matsui, J. K.; Molander, G. A. J. Am. Chem. Soc. 2017, 139, 12251. |
| [34] | Matsui, J. K.; Gutiérrez-Bonet, á.; Rotella, M.; Alam, R.; Gutierrez, O.; Molander, G. A. Angew. Chem., Int. Ed. 2018, 57, 15847. |
| [35] | For selected examples see: (a) Gong, X.; Wang, M.; Ye, S.; Wu, J . Org. Lett. 2019, 21, 1156. |
| [35] | (b) Ye, S.; Qiu, G.; Wu, J . Chem. Commun. 2019, 55, 1013. |
| [35] | (c) Ye, S.; Zheng, D.; Wu, J.; Qiu, G. Chem. Commun. 2019, 55, 2214. |
| [35] | (d) Ye, S.; Li, Y.; Wu, J.; Li, Z. Chem. Commun. 2019, 55, 2489. |
| [35] | (e) Gong, X.; Li, X.; Xie, W.; Wu, J.; Ye, S. Org. Chem. Front. 2019, 6, 1863. |
| [35] | (f) Zhang, J.; Xie, W.; Ye, S.; Wu, J. Org. Chem. Front. 2019, 6, 2254. |
| [35] | (g) Ye, S.; Xiang, T.; Li, X.; Wu, J. Org. Chem. Front. 2019, 6, 2183. |
| [35] | (h) Ye, S.; Li, X.; Xie, W.; Wu, J. Asian J. Org. Chem. 2019, 8, 893. |
| [35] | (i) Ye, S.; Li, X.; Xie, W.; Wu, J. Eur. J. Org. Chem. 2019, 10.1002/ejoc.201900396. |
| [35] | (j) Zhang, J.; Li, X.; Xie, W.; Ye, S.; Wu, J. Org. Lett. 2019, 21, DOI: 10.1021/acs.orglett.9b01323. |
| [35] | (k) Zong, Y.; Lang, Y.; Yang, M.; Li, X.; Fan, X.; Wu, J. Org. Lett. 2019, 21, 1935. |
| [36] | Wang, X.; Li, H.; Qiu, G.; Wu, J . Chem. Commun. 2019, 55, 2062. |
| [37] | Wang, X.; Yang, M.; Xie, W.; Fan, X.; Wu, J . Chem. Commun. 2019, 55, 6010. |
| [38] | Verrier, C.; Alandini, N.; Pezzetta, C.; Moliterno, M.; Buzzetti, L.; Hepburn, H. B.; Vega-Penaloza, A.; Silvi, M.; Melchiorre, P . ACS Catal. 2018, 8, 1062. |
| [39] | Goti, G.; Bieszczad, B.; Vega-Penaloza, A.; Melchiorre, P. Angew. Chem., Int. Ed. 2019, 58, 1213. |
| [40] | de Assis, F. F.; Huang, X.; Akiyama, M.; Pilli, R. A.; Meggers, E. J. Org. Chem. 2018, 83, 10922. |
| [41] | Zhang, H.-H.; Zhao, J.-J.; Yu, S. J. Am. Chem. Soc. 2018, 140, 16914. |
| [42] | Li, F.; Tian, D.; Fan, Y.; Lee, R.; Lu, G.; Yin, Y.; Qiao, B.; Zhao, X.; Xiao, Z.; Jiang, Z . Nat. Commun. 2019, DOI: 10.1038/s41467-019- 09857-9. |
/
| 〈 |
|
〉 |