

Visible-Light Photocatalytic Remote Thiolation of Aldehydes Triggered by Sulfonylation of Alkenes With Thiosulfonates
Received date: 2019-05-02
Online published: 2019-06-13
Supported by
Project supported by the National Natural Science Foundation of China(No. 21672191)
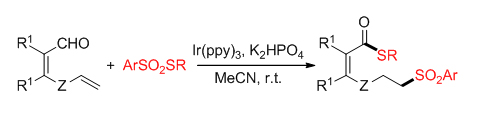
Due to the prevalence of organosulfur compounds in pharmaceuticals, agrochemicals, and functional materials, the development of new efficient and practical methods for the construction of C—S bonds is highly desirable in organic synthesis. Recently, the radical sulfonylation of alkenes has attracted considerable attention because of its efficient and versatile synthesis of organosulfur compounds under mild reaction conditions. The previous methods usually involve the formation of one C—S bond. In contrast, the thiosulfonylation of alkenes represents a highly attractive protocol for the concurrent formation of two distinct C—S bonds. Herein, a novel visible-light photocatalytic remote thiolation of aldehydes triggered by the radical sulfonylation of unactivated alkenes has been developed, with readily available thiosulfonates as both the sulfonating and thiolating reagents, successfully giving 6- or 7-sulfonylated thioesters in moderate to high yields with broad substrate scope and excellent atom-economics. As compared to the traditional methods that are limited to 1,2- or 1,1-thiosulfonylation of alkenes, the reaction described here constitutes the first example of 1,6- or 1,7-thiosulfonylation of functionalized alkenes, thus offering a good complementary protocol to the existing methods. Preliminary mechanistic studies suggest a radical pathway consisting of the formation of sulfonyl radical, alkene sulfonylation, intramolecular 1,n-hydrogen atom transfer (1,n-HAT), and thiolation of acyl radical. A representative procedure for the visible-light induced remote thiolation of aldehydes initiated by the sulfonylation of alkenes with thiosulfonates is as following: To a mixture of thiosulfonates 2 (0.5 mmol), Ir(ppy)3 (1 mol%), and K2HPO4 (0.5 mmol) in 4 mL of MeCN was added alkenyl aldehydes 1 (0.25 mmol) under a N2 atmosphere. After 18 h of irradiation with 15 W blue LEDs at 25 ℃, the reaction mixture was quenched with water, extracted with EtOAc, dried over anhydrous Na2SO4, concentrated, and purified by column chromatography with silica gel (EtOAc/petroleum ethers=1∶5) to give products 3 or 4.
Junhang Yang, , Xiaobo Fu, , Zenghui Lu, , Gangguo Zhu, . Visible-Light Photocatalytic Remote Thiolation of Aldehydes Triggered by Sulfonylation of Alkenes With Thiosulfonates[J]. Acta Chimica Sinica, 2019 , 77(9) : 901 -905 . DOI: 10.6023/A19050161
| [1] | Madasu, S. B.; Vekariya, N. A.; Kiran, M. N. V. D. H.; Gupta, B.; Islam, A.; Douglas, P. S.; Babu, K. R . Beilstein J. Org. Chem. 2012, 8, 1400. |
| [2] | Fromtling, R. A . Drugs Future 1989, 14, 1165. |
| [3] | Calverley, P. M. A.; Anderson, J. A.; Celli, B.; Ferguson, G. T.; Jenkins, C.; Jones, P. W.; Yates, J. C.; Vestbo, J . N. Engl. J. Med. 2007, 356, 775. |
| [4] | (a) Chen, X.; Gan, X.; Chen, J.; Chen, Y.; Wang, Y.; Hu, D.; Song, B . Chin. J. Org. Chem. 2017, 37, 2343. |
| [4] | ( 陈学文, 甘秀海, 陈吉祥, 陈永中, 王艳娇, 胡德禹, 宋宝安 . 有机化学, 2017, 37, 2343.) |
| [4] | (b) Chen, Y.; Wang, S.; Jiang, Q.; Cheng, C.; Xiao, X.; Zhu, G . J. Org. Chem. 2018, 83, 716. |
| [4] | For a review, see:(c) Feng, M.; Tang, B.; Liang, S. H.; Jiang, X . Curr. Top. Med. Chem. 2016, 16, 1200 and references cited therein. |
| [5] | Julia, M.; Paris, J. M . Tetrahedron Lett. 1973, 14, 4833. |
| [6] | Olah, G. A.; Mathew, T.; Prakash, G. K. S . Chem. Commun. 2001,1696. |
| [7] | (a) Deeming, A. S.; Russell, C. J.; Hennessy, A. J.; Willis, M. C . Org. Lett. 2014, 16, 150. |
| [7] | (b) Wan, Y.; Zhang, J.; Chen, Y.; Kong, L.; Luo, F.; Zhu, G . Org. Biomol. Chem. 2017, 15, 7204. |
| [8] | (a) Zhou, Q.; Gui, J.; Pan, C.-M.; Albone, E.; Cheng, X.; Suh, E. M.; Grasso, L.; Ishihara, Y.; Baran, P. S . J. Am. Chem. Soc. 2013, 135, 12994. |
| [8] | (b) Miao, W.; Zhao, Y.; Ni, C.; Gao, B.; Zhang, W.; Hu, J . J. Am. Chem. Soc. 2018, 140, 880. |
| [8] | (c) Griffiths, R. J.; Kong, W. C.; Richards, S. A.; Burley, G. A.; Willis, M. C.; Talbot, E. P. A . Chem. Sci. 2018, 9, 2295. |
| [9] | For selected reviews see: (a) Deeming, A. S.; Emmett, E. J.; Richards-Taylor, C. S.; Willis, M. C. Synthesis. 2014, 46, 2701. |
| [9] | (b) Liu, N.-W.; Liang, S.; Manolikakes, G. Synthesis. 2016, 48, 1939. |
| [9] | (c) Qiu, G.; Lai, L.; Cheng, J.; Wu, J . Chem. Commun. 2018, 54, 10405. |
| [9] | (d) Guo, W.; Tao, K.; Tan, W.; Zhao, M.; Zheng, L.; Fan, X . Org. Chem. Front.. 2019, 5, DOI: 10.1039/c8qo01353e. |
| [9] | (e) Wang, S.; Zheng, Q.; Duan, P.; Liu, W . Chin. J. Org. Chem.. 2017, 37, 1653. |
| [9] | ( 王守锋, 郑庆飞, 段盼盼, 刘文 , 有机化学, 2017, 37, 1653.) |
| [9] | (f) Tan, F.; Xiao, W.; Zeng, G . Chin. J. Org. Chem.. 2017, 37, 824. |
| [9] | ( 谭芬, 肖文精, 曾国平 , 有机化学, 2017, 37, 824.) |
| [9] | (g) Li, S.; Hong, H.; Han, L.; Zhang, T.; Wang, Y.; Zhu, N . Chin. J. Org. Chem.. 2018, 38, 304. |
| [9] | ( 李闪闪, 洪海龙, 韩利民, 张田苗, 王云龙, 竺宁, , 有机化学, 2018, 38, 304.) |
| [9] | (h) Li, S.-S.; Wang, J . Acta Chim. Sinica . 2018, 76, 913. |
| [9] | ( 李树森, 王剑波 , 化学学报, 2018, 76, 913.) |
| [9] | For a report, see:(i) Yuan, Y.; Cao, Y.; Qiao, J.; Lin, Y.; Jiang, X.; Weng, Y.; Tang, S.; Lei, A . Chin. J. Chem. 2019, 37, 49. |
| [10] | (a) Liu, T.; Li, Y.; Lai, L.; Cheng, J.; Sun, J.; Wu, J. Org. Lett. 2018, 20, 3605. |
| [10] | (b) Ye, S.; Zheng, D.; Wu, J.; Qiu, G. Chem. Commun. 2019, 55, 2214. |
| [11] | (a) Meyer, A. U.; J?ger, S.; Hari, D. P.; K?nig, B . Adv. Synth. Catal. 2015, 357, 2050. |
| [11] | (b) Zhang, G.; Zhang, L.; Yi, H. Luo, Y.; Qi, X.; Tung, C.-H.; Wu, L.-Z.; Lei, A . Chem. Commun. 2016, 52, 10407. |
| [11] | (c) Ratushnyy, M.; Kamenova, M.; Gevorgyan, V. Chem. Sci. 2018, 9, 7193. |
| [11] | (d) Sun, D.; Zhang, R . Org. Chem. Front. 2018, 5, 92. |
| [11] | (e) Cai, S.; Xu, Y.; Chen, D.; Li, L.; Chen, Q.; Huang, M.; Weng, W . Org. Lett. 2016, 128, 7440. |
| [12] | (a) Quebatte, L.; Thommes, K.; Severin, K . J. Am. Chem. Soc. 2006, 128, 7440. |
| [12] | (b) Hossain, A.; Engl, S.; Lutsker, E.; Reiser, O . ACS Catal. 2019, 9, 1103. |
| [12] | (c) Taniguchi, T.; Idota, A.; Ishibashi, H . Org. Biomol. Chem. 2011, 9, 3151. |
| [12] | (d) Pagire, S. K.; Paria, S.; Reiser, O . Org. Lett. 2016, 18, 2016 |
| [12] | (e) Xiong, Y.; Sun, Y.; Zhang, G . Org. Lett. 2018, 20, 6250. |
| [12] | (f) Rao, W.-H.; Jiang, L.-L.; Liu, X.-M.; Chen, M.-J.; Chen, F.-Y.; Jiang, X.; Zhao, J.-X.; Zou, G.-D.; Zhou, Y.-Q.; Tang, L . Org. Lett. 2019, 21, 2890. |
| [12] | (g) Wang, H.; Wang, G.; Lu, Q.; Chiang, C.-W.; Peng, P.; Zhou, J.; Lei, A . Chem. Eur. J. 2016, 22, 14489. |
| [12] | (h) Yuan, Y.; Cao, Y.; Lin, Y.; Li, Y.; Huang, Z.; Lei, A . ACS Catal. 2018, 8, 10871. |
| [13] | (a) Gao, Y.; Mei, H.; Han, J.; Pan, Y . Chem. Eur. J. 2018, 24, 17205. |
| [13] | (b) Sun, J.; Li, P.; Guo, L.; Yu, F.; He, Y.-P.; Chu, L. Chem. Commun. 2018, 54, 3162. |
| [13] | (c) Pirenne, V.; Kurtay, G.; Voci, S.; Bouffier, L.; Sojic, N.; Robert, F.; Bassani, D. M.; Landais, Y . Org. Lett. 2018, 20, 4521. |
| [14] | (a) Chen, Z.-Z.; Liu, S.; Hao, W.-J.; Xu, G.; Wu, S.; Miao, J.-N.; Jiang, B.; Wang, S.-L.; Tu, S.-J.; Li, G . Chem. Sci. 2015, 6, 6654. |
| [14] | (b) Huang, M.-H.; Zhu, C.-F.; He, C.-L.; Zhu, Y.-L.; Hao, W.-J.; Wang, D.-C.; Tu, S.-J.; Jiang, B . Org. Chem. Front. 2018, 5, 1643. |
| [14] | (c) Wu, W.; Yi, S.; Yu, Y.; Huang, W.; Jiang, H . J. Org. Chem. 2017, 82, 1224. |
| [14] | (d) Cao, X.; Cheng, X.; Xuan, J . Org. Lett. 2018, 20, 449. |
| [15] | (a) Zhu, D.; Shao, X.; Hong, X.; Lu, L.; Shen, Q . Angew. Chem., Int. Ed. 2016, 55, 15807. |
| [15] | (b) Zhao, Q.; Lu, L.; Shen, Q . Angew. Chem., Int. Ed. 2017, 56, 11575. |
| [16] | (a) Li, H.; Shan, C.; Tung, C.-H.; Xu, Z. Chem. Sci. 2017, 8, 2610. |
| [16] | (b) Huang, S.; Thirupathi, N.; Tung, C.-H.; Xu, Z . J. Org. Chem. 2018, 83, 9449. |
| [17] | He, F.-S.; Wu, Y.; Zhang, J.; Xia, H.; Wu, J . Org. Chem. Front. 2018, 5, 2940. |
| [18] | (a) Cheng, C.; Liu, S.; Lu, D.; Zhu, G. Org. Lett. 2016, 18, 2852. |
| [18] | (b) Nie, X.; Cheng, C.; Zhu, G . Angew. Chem., Int. Ed. 2017, 56, 1898. |
| [18] | (c) Jin, W.; Zhou, Y.; Zhao, Y.; Ma, Q.; Kong, L.; Zhu, G . Org. Lett. 2018, 20, 1435. |
| [18] | (d) Wan, Y.; Shang, T.; Lu, Z. Zhu, G . Org. Lett. 2019, 21, 4187. |
| [19] | For selected reviews on photocatalysis, see:(a) Narayanam, J. M. R.; Stephenson, C. R. J . Chem. Soc. Rev. 2011, 40, 102. |
| [19] | (b) Xuan, J.; Xiao, W.-J . Angew. Chem., Int. Ed. 2012, 51, 6828. |
| [19] | (c) Prier, C. K.; Rankic, D. A.; MacMillan, D. W. C . Chem. Rev. 2013, 113, 5322. |
| [19] | (d) Xi, Y.; Yi, H.; Lei, A . Org. Biomol. Chem. 2013, 11, 2387. |
| [19] | (e) Yu, S.; Zhang, Y.; Wang, R.; Jiang, H.; Cheng, Y.; Kadi, A.; Fun, H.-K . Synthesis. 2014, 2711. |
| [19] | (f) Xie, J.; Jin, H.; Xu, P.; Zhu, C . Tetrahedron Lett. 2014, 55, 36. |
| [19] | (g) Wang, C.; Lu, Z . Org. Chem. Front. 2015, 2, 179. |
| [19] | (h) Matsui, J. K.; Lang, S. B.; Heitz, D. R.; Molander, G. A . ACS Catal. 2017, 7, 2563. |
| [20] | For selected reviews see: (a) Hu, X.-Q.; Chen, J.-R.; Xiao, W.-J . Angew. Chem., Int. Ed. 2017, 56, 1960. |
| [20] | (b) Li, W.; Xu, W.; Xie, J.; Yu, S.; Zhu, C . Chem. Soc. Rev. 2018, 47, 654. |
| [20] | (c) Stateman, L. M.; Nakafuku, K. M.; Nagib, D. A . Synjournal 2018, 50, 1569. |
| [20] | (d) Nechab, M.; Mondal, S.; Bertrand, M. P . Chem. Eur. J.. 2018, 20, 16034. |
| [20] | For selected reports involving 1,n-HAT since 2018, see:(e) Short, M. A.; Blackburn, J. M.; Roizen, J. L . Angew. Chem., Int. Ed. 2018, 57, 296. |
| [20] | (f) Dauncey, E. M.; Morcillo, S. P.; Douglas, J. J.; Sheikh, N. S.; Leonori, D . Angew. Chem., Int. Ed. 2018, 57, 744. |
| [20] | (g) Wu, X.; Wang, M.; Huan, L.; Wang, D.; Wang, J.; Zhu, C . Angew. Chem., Int. Ed. 2018, 57, 1640. |
| [20] | (h) Wu, S.; Wu, X.; Wang, D.; Zhu, C . Angew. Chem., Int. Ed. 2019, 58, 1499. |
| [20] | (i) Jiang, H.; Studer, A . Angew. Chem., Int. Ed. 2018, 57, 1692. |
| [20] | (j) Xia, Y.; Wang, L.; Studer, A . Angew. Chem., Int. Ed. 2018, 57, 12940. |
| [20] | (k) Ratushnyy, M.; Parasram, M.; Wang, Y.; Gevorgyan, V . Angew. Chem., Int. Ed. 2018, 57, 2712. |
| [20] | (l) Chuentragool, P.; Yadagiri, D.; Morita, T.; Sarkar, S.; Parasram, M.; Wang, Y.; Gevorgyan, V . Angew. Chem., Int. Ed. 2019, 58, 1794. |
| [20] | (m) Na, C. G.; Alexanian, E. J . Angew. Chem., Int. Ed. 2018, 57, 13106. |
| [20] | (n) Li, Z.; Wang, Q.; Zhu, J . Angew. Chem., Int. Ed. 2018, 57, 13288. |
| [20] | (o) Bao, X.; Wang, Q.; Zhu, J . Angew. Chem., Int. Ed. 2019, 58, 2139. |
| [20] | (p) Kim, I.; Park, B.; Kang, G.; Kim, J.; Jung, H.; Lee, H.; Baik, M.-H.; Hong, S . Angew. Chem., Int. Ed. 2018, 57, 15517. |
| [20] | (q) Guan, H.; Sun, S.; Mao, Y.; Chen, L.; Lu, R.; Huang, J.; Liu, L . Angew. Chem., Int. Ed. 2018, 57, 11413. |
| [20] | (r) Hu, A.; Guo, J.-J.; Pan, H.; Tang, H.; Gao, Z.; Zuo, Z . J. Am. Chem. Soc. 2018, 140, 1612. |
| [20] | (s) An, X.-D.; Jiao, Y.-Y.; Zhang, H.; Gao, Y.; Yu, S . Org. Lett. 2018, 20, 401. |
| [20] | (t) Zhu, Y.; Huang, K.; Pan, J.; Qiu, X.; Luo, X.; Qin, Q.; Wei, J.; Wen, X.; Zhang, L.; Jiao, N . Nat. Commun. 2018, 9, 2625. |
| [20] | (u) Li, G.-X.; Hu, X.; He, G.; Chen, G . Chem. Sci. 2019, 10, 688. |
| [20] | (v) Zhang, Z.; Stateman, L. M.; Nagib, D. A . Chem. Sci. 2019, 10, 1207. |
| [20] | (w) Wu, K.; Wang, L.; Colón-Rodríguez, S.; Flechsig, G.-U.; Wang, T . Angew. Chem., Int. Ed. 2019, 58, 1774. |
| [20] | (x) Liu, Z.; Xiao, H.; Zhang, B.; Shen, H.; Zhu, L.; Li, C . Angew. Chem., Int. Ed. 2019, 58, 2510. |
/
| 〈 |
|
〉 |