

Radical-Promoted Cross Dehydrogenative Coupling of Ketones and Esters with Electron-Rich Heteroarenes
Received date: 2019-05-21
Online published: 2019-06-21
Supported by
Project supported by the National Natural Science Foundation of China(No.21672089);the State Key Laboratory of Applied Organic Chemistry of Lanzhou University and the Nanjing University of Chinese Medicine
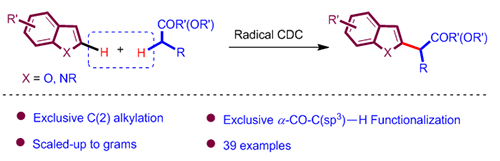
The cross dehydrogenative coupling (CDC) via highly selective C—H bond functionalization represents one of the most atom-economical, environmentally-benign and efficient synthetic strategies. For a long time, the cleavage of C—H bonds initiated by free radicals has been regarded as unselective and useless. However, more and more studies have shown that free radical mediated strategies could also achieve C—H bond functionalization in high selectivity recently. In general, it’s well-known that nucleophilic free radical species tend to extract hydrogen atoms on electron-deficient C—H bonds, while electrophilic free radicals abstract hydrogen atoms on electron-rich C—H bonds. A recent study by our group shows that after thermal decomposition of peroxy tert-butyl ether, the electron-rich methyl radicals are produced. Then the radical cleavage of the C(sp 3)—H bond in ketone/ester would happen prior to the α-carbonyl-C—H bond. Subsequently, the electrophilic α-carbonyl-C-centered radical selectively reacted with electron-rich olefins to afford new C—C bonds. Here, a free-radical initiated highly selective cross dehydrogenative coupling reaction of simple ketones and esters with electron-rich heteroarenes was demonstrated. The ketones and esters were used as solvent, and they would afford the corresponding α-carbonyl C-centered radicals, which then add to heteroaromatics leading to a series of C(2)-functionalized heterocycles. The chemoselectivity of this system was well-controlled by application of the polar effect of free radicals. In addition, this protocol features fast, simple operation, good functional group tolerance and site specific etc. The potential of this method was demonstrated through the synthesis of non-steroidal anti-inflammatory and analgesic drug tolmetin. It is expected to have wide applications in synthetic organic chemistry. Typical reaction conditions are as follows: a mixture of heteroarenes (1 equiv., 0.20 mmol), TBPA (3 equiv., 0.06 mmol) and ketones/esters (6 mL) was heated under reflux at 130 ℃ for about 1 h. After completion of the reaction, the crude product was cooled to room temperature, the excess solvent was recovered by rotary evaporator and the residue was further purified by column chromatography on silica gel to obtain the desired product (eluent: petroleum ether/ethyl acetate).
Yingxia Xiao, , Zhong-Quan Liu, . Radical-Promoted Cross Dehydrogenative Coupling of Ketones and Esters with Electron-Rich Heteroarenes[J]. Acta Chimica Sinica, 2019 , 77(9) : 874 -878 . DOI: 10.6023/A19050189
| [1] | For selected recent reviews on CDC reactions, see: (a) Li, C.-J . Acc. Chem. Res. 2009, 42, 335. |
| [1] | (b) Yeung, C. S.; Dong, V. M. Chem. Rev. 2011, 111, 1215. |
| [1] | (c) Girard, S. A.; Knauber, T.; Li, C.-J . Angew. Chem., Int. Ed 2014, 53, 74. |
| [1] | (d) Jia, F.; Li, Z . Org. Chem. Front 2014, 1, 194. |
| [1] | (e) Zhang, J.; Lu, Q.; Liu, C.; Lei, A . Chin. J. Org. Chem 2015, 35, 743. |
| [1] | (张剑, 陆庆全, 刘超, 雷爱文, 有机化学, 2015, 35, 743.); |
| [1] | (f) Zhang, G.; Bian, C.; Lei, A . Chin. J. Catal 2015, 36, 1428. |
| [1] | (g) Pei, P.; Zhang, F.; Yi, H.; Lei, A . Acta Chim. Sinica 2017, 75, 15. |
| [1] | (裴朋昆, 张凡, 易红, 雷爱文, 化学学报, 2017, 75, 15.); |
| [1] | (h) Shao, A.; Li, N.; Gao, Y.; Zhan, J.; Chiang, C. W.; Lei, A . Chin. J. Chem. 2018, 36, 619; |
| [1] | (i) Liu, Y.; Yi, H.; Lei, A . Chin. J. Chem. 2018, 36, 692. |
| [2] | For selected recent reviews on C—H functionalization, see: (a) Zhang, S.; Zhang, F.; Tu, Y.-Q. Chem. Soc. Rev. 2011, 40, 1937. |
| [2] | (b) Davies, H. M. L.; Morton, D. Chem. Soc. Rev. 2011, 40, 1857. |
| [2] | (c) Newhouse, T.; Baran, P. S . Angew. Chem., Int. Ed. 2011, 50, 3362. |
| [2] | (d) Liu, C.; Zhang, H.; Shi, W.; Lei, A . Chem. Rev. 2011, 111, 1780. |
| [2] | (e) Engle, K. M.; Mei, T.-S.; Wasa, M.; Yu, J.-Q . Acc. Chem. Res. 2012, 45, 788. |
| [2] | (f) Roizen, J. L.; Harvey, M. E.Du Bois, J . Acc. Chem. Res. 2012, 45, 911. |
| [2] | (g) Rouquet, G.; Chatani, N . Angew. Chem., Int. Ed. 2013, 52, 11726. |
| [2] | (h) He, J.; Wasa, M.; Chan, K. S. L.; Shao, Q.; Yu, J.-Q . Chem. Rev. 2017, 117, 8754. |
| [2] | (i) Le Bras, J.; Muzart, J . Chem. Rev. 2011, 111, 1170. |
| [2] | (j) Sun, C.-L.; Li, B.-J.; Shi, Z.-J . Chem. Rev. 2011, 111, 1293. |
| [2] | (k) Cho, S. H.; Kim, J. Y.; Kwak, J.; Chang, S . Chem. Soc. Rev. 2011, 40, 5068. |
| [2] | (l) Shang, X.; Liu, Z.-Q . Chem. Soc. Rev. 2013, 42, 3253; |
| [2] | (m) Yang, L.; Huang, H . Chem. Rev. 2015, 115, 3468. |
| [2] | (n) Guo, X.-X.; Gu, D.-W.; Wu, Z.; Zhang, W . Chem. Rev. 2015, 115, 1622. |
| [2] | (o) Zheng, Q.-Z.; Jiao, N . Chem. Soc. Rev. 2016, 45, 4590. |
| [2] | (p) Murakami, K.; Yamada, S.; Kaneda, T.; Itami, K . Chem. Rev. 2017, 117, 9302. |
| [2] | (q) Yuan, S.; Wang, Y.; Qiu, G.; Liu, J . Chin. J. Org. Chem. 2017, 37, 566. |
| [2] | (袁斯甜, 王艳华, 邱观音生, 刘晋彪, 有机化学, 2017, 37, 566.); |
| [2] | (r) Zhang, J. J.; Cheng, Y. B.; Duan, X. H . Chin. J. Chem. 2017, 35, 311. |
| [2] | (s) Ruan, L.; Chen, C.; Zhang, X.; Sun, J . Chin. J. Org. Chem. 2018, 38, 3155. |
| [2] | (阮利衡, 陈春欣, 张晓欣, 孙京, 有机化学, 2018, 38, 3155.); |
| [2] | (t) Zhang, X.; Li, P.; Yuan, Y.; Jia, X . Chin. J. Org. Chem. 2018, 38, 2435. |
| [2] | (张学文, 李鹏飞, 袁宇, 贾晓东, 有机化学, 2018, 38, 2435.); |
| [2] | (u) Gu, Z.; Ji, S . Acta. Chim. Sinica 2018, 76, 347. |
| [2] | (顾正祥, 纪顺俊, 化学学报, 2018, 76, 347.) |
| [3] | For selected recent reviews, see: (a) Shang, X.; Liu, Z.-Q . Acta Chim. Sinica 2015, 73, 1275. |
| [3] | ( 尚筱洁, 柳忠全 , 化学学报, 2015, 73, 1275.) |
| [3] | (b) Yi, H.; Zhang, G.; Wang, H.; Huang, Z.; Wang, J.; Singh, A. K.; Lei, A. Chem. Rev. 2017, 117, 9016. |
| [4] | (a) Harris, E. F. P.; Waters, W. A . Nature 1952, 170, 212. |
| [4] | (b) Walling, C. Pure Appl. Chem. 1967, 15, 69. |
| [4] | (c) Tedder, J. M. Angew. Chem. Int. Ed. Engl. 1982, 21, 401. |
| [4] | (d) Giese, B . Angew. Chem. Int. Ed. Engl. 1989, 28, 969. |
| [4] | (e) Roberts, B. P . Chem. Soc. Rev. 1999, 28, 25. |
| [5] | Ravelli, D.; Fagnoni, M.; Fukuyama, T.; Nishikawa, T.; Ryu, I . ACS Catal. 2018, 8, 701. |
| [6] | Tian, Y.; Sun, C.; Tan, R. X.; Liu, Z.-Q . Green Chem. 2018, 20, 588. |
| [7] | (a) Snider, B. B. Chem. Rev. 1996, 96, 339. |
| [7] | (b) Heiba E. I.; Dessau, R. M. J. Am. Chem. Soc. 1971, 93, 524. |
| [7] | (c) Iwahama, T.; Sakaguchi, S. Ishii, Y. Chem. Commun. 2000, 2317. |
| [7] | (d) Linker, U.; Kersten, B.; Linker, T . Tetrahedron 1995, 51, 9917. |
| [7] | (e) Xie, J.; Huang, Z.-Z . Chem. Commun. 2010, 46, 1947. |
| [7] | (f) Zhu, L.; Chen, H.; Wang, Z.; Li, C . Org. Chem. Front. 2014, 1, 1299. |
| [7] | (g) Schweitzer-Chaput, B.; Demaerel, J.; Engler, H.; Klussmann, M . Angew. Chem., Int. Ed. 2014, 53, 8737. |
| [7] | (h) Chu, X.; Meng, H.; Zi, Y.; Xu, X.-P.; Ji, S.-J . Chem. -Eur. J. 2014, 20, 17198. |
| [7] | (i) Lan, X.; Wang, N.-X.; Zhang, W.; Wen, J.; Bai, C.; Xing, Y.-L.; Li, Y.-H . Org. Lett. 2015, 17, 4460. |
| [7] | (j) Shiraishi, Y.; Tsukamoto, D.; Hirai, T . Org. Lett. 2008, 10, 3117. |
| [7] | (k) Tsukamoto, D.; Shiraishi, Y.; Hirai, T . J. Org. Chem. 2010, 75, 1450. |
| [8] | (a) Liu, Z.-Q.; Li, Z. Chem . Commun. 2016, 52, 14278. |
| [8] | (b) Xu, Z.; Hang, Z.; Chai, L.; Liu, Z.-Q. Org . Lett. 2016, 18, 4662. |
/
| 〈 |
|
〉 |