

Effect of K-Doping on the Sodium-storage Performance of Sodium Vanadate Nanoplates
Received date: 2019-01-23
Online published: 2019-07-02
Supported by
Project supported by the National Natural Science Foundation of China (Nos. 51572194, 51672189), and the Hunan Provincial Natural Science Foundation (Nos. 2018JJ2386, 2018JJ2393).
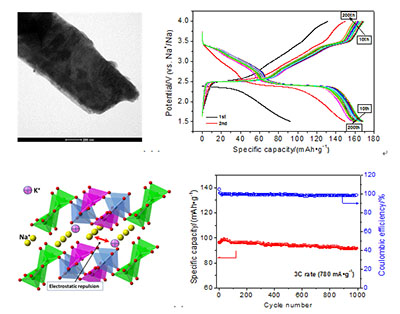
Na-ion batteries with lower cost than Li-ion batteries would be developed to large-scale energy-storage device to store solar and wind energies. However, large radius renders Na+ ions to insert into/extract out the layered transition metal oxides (LTMOs) sluggishly. To improve the intercalation dynamics of Na+ ions, the interlayer spacing of crystals has to be expanded for those LTMOs that are capable of fast lithiation and delithiation. Herein, a LTMO based on vanadium is firstly doped with larger K+ ions to expand the interlayer spacing to yield K+-doped sodium vanadate (Na5KxV12O32) cathode material by a hydrothermal method at 200℃ for 24 h followed by calcination at 500℃ for 3 h. The samples were characterized by scanning electron microscope (SEM)/transmission electron microscope (TEM), X-ray diffraction (XRD) and X-ray photoelectron spectroscopy (XPS) technologies. Effect of the doping amount of K+ on the structure and sodium-storage performance of the sample was studied in detail. The synthesized materials display nanoplate morphology viewing from the TEM images. K+ ions are doped into the interlayer of the sodium vanadate crystallites, which is proved by analysis of the XRD patterns and XPS spectra. Expanded interlayer spacing favors Na+ ions' intercalation and deintercalation between the[V3O8]- layers, which is testified by the chemical diffusion coefficient of cathodes, thus enhancing the rate capability. On the other hand, the chemically pre-intercalated K+ ions are pinned in the crystallites during insertion and extraction of Na+ ions and act as pillars to stabilize the layered structure, improving the cycliability of the cathode. However, excessive doping of K+ leads to a discounted rate capability of the cathode, suggesting an optimized amount of K+ doping into the crystal. The results from galvanostatic charge-discharge tests indicate that the obtained NVO(3K) sample, in which 0.118 mol of K+ ions are doped into per mol of Na5V12O32, presents the best electrochemical performance among the various samples. It can deliver the maximum capacities of 169, 160, 148, 132, 98 and 69 mAh·g-1 at the rates of 0.1C, 0.2C, 0.5C, 1C, 3C and 10C after activated for several times over the voltage window of 4.0~1.5 V (vs. Na+/Na), respectively. Even run at 3C rate, it can retain 93.0% of the maximum capacity after 1000 cycles, exhibiting excellent rate capability and stable cycliability. The results suggest that doping of K+ ions into the interlayer of crystallites can significantly improving the rate capability as well as cycling performance of the obtained Na5V12O32. Our investigation demonstrates that design of K+-doped sodium vanadate cathode materials is beneficial for harvesting superior performance, of which the Na5K0.118V12O32 nanoplates can be developed into a novel cathode material for sodium-ion batteries in the future.
Song Xuexi , Li Jicheng , Li Zhaohui , Li Xifei , Ding Yanhuai , Xiao Qizhen , Lei Gangtie . Effect of K-Doping on the Sodium-storage Performance of Sodium Vanadate Nanoplates[J]. Acta Chimica Sinica, 2019 , 77(7) : 625 -633 . DOI: 10.6023/A19010040
[1] Xiang, X. D.; Lu, Y. Y.; Chen, J. Acta Chim. Sinica 2017, 75, 154(in Chinese). (向兴德, 卢艳莹, 陈军, 化学学报, 2017, 75, 154.)
[2] Yabuuchi, N.; Kubota, K.; Dahbi, M.; Komaba, S. Chem. Rev. 2014, 114, 11636.
[3] Ni, J. F.; Fu, S.; Yuan, Y. F.; Ma, L.; Jiang, Y.; Li, L.; Lu, J. Adv. Mater. 2018, 30, 1704337.
[4] Chen, J.; Li, L.; Wu, L.; Yao, Q.; Yang, H.; Liu, Z.; Xia, L.; Chen, Z.; Duan, J.; Zhong, S. J. Power Sources 2018, 406, 110.
[5] Bai, Q.; Yang, L. F.; Chen, H. L.; Mo, Y. F. Adv. Energy Mater. 2018, 8, 1702998.
[6] Fang, Y. J.; Chen, Z. X.; Ai, X. P.; Yang, H. X.; Cao, Y. L. Acta Phys.-Chim. Sin. 2017, 33, 211(in Chinese). (方永进, 陈重学, 艾新平, 杨汉西, 曹余良, 物理化学学报, 2017, 33, 211.)
[7] Lu, Y. X.; Zhao, C. L.; Rong, X. H.; Chen, L. Q.; Hu, Y. S. Acta Phys. Sin. 2018, 67, 120601(in Chinese). (陆雅翔, 赵成龙, 容晓晖, 陈立泉, 胡勇胜, 物理学报, 2018, 67, 120601.)
[8] Rong, X. H.; Liu, J.; Hu, E. Y.; Liu, Y. J.; Wang, Y.; Wu, J. P.; Yu, X. Q.; Page, K. A.; Hu, Y. S.; Yang, W. L.; Li, H.; Yang, X. Q.; Chen, L. Q.; Huang, X. J. Joule 2018, 2, 125.
[9] Wang, W. J.; Zhu, W. H.; Zhang, Y. J.; Liu, Y. J.; Zhang, Q.; Fu, L. Chin. J. Chem. 2018, 36, 866.
[10] Wang, L.; Yang, G. R.; Wang, J. N.; Wang, S. L.; Peng, S. J.; Yan, W. Acta Chim. Sinica 2018, 76, 666(in Chinese). (王玲, 杨国锐, 王嘉楠, 王思岚, 彭生杰, 延卫, 化学学报, 2018, 76, 666.)
[11] Li, P.; Liu, J.; Sun, W.; Tao, Z.; Chen, J. Acta Chim. Sinica 2018, 76, 286(in Chinese). (李攀, 刘建, 孙惟袆, 陶占良, 陈军, 化学学报, 2018, 76, 286.)
[12] Cao, X. Y.; Yang, Q.; Zhu, L. M.; Xie, L. L. Ionics 2018, 24, 1.
[13] Lu, Y. K.; Wu, J.; Liu, J.; Lei, M.; Tang, S. S.; Lu, P. J.; Yang, L. Y.; Yang, H. R.; Yang, Q. ACS Appl. Mater. Interfaces 2015, 7, 17433.
[14] Cai, Y.; Zhou, J.; Fang, G.; Cai, G.; Pan, A.; Liang, S. J. Power Sources 2016, 328, 241.
[15] Cai, Y. S.; Zhou, J.; Fang, G. Z.; Cai, G. M.; Pan, A. Q.; Liang, S. Q. RSC Adv. 2017, 7, 29481.
[16] Kang, H. Y.; Liu, Y. C.; Shang, M. H.; Lu, T. Y.; Wang, Y. J.; Jiao, L. F. Nanoscale 2015, 7, 9261.
[17] Lu, Y. K.; Su, N.; Cheng, L. Z.; Liu, J.; Yang, L. Y.; Yang, H. R.; Yang, Q.; Li, S.; Min, J.; Lei, M. Mater. Lett. 2016, 183, 346.
[18] Mei, P.; Wu, X. L.; Xie, H. M. Sun, L. Q.; Zeng, Y. P.; Zhang, J. P.; Tai, L. H.; Guo, X.; Cong, L.; Ma, S. C.; Yao, C.; Wang, R. C. RSC Adv. 2014, 4, 25494.
[19] Sun, D.; Xu, G. Q.; Wang, H. Y.; Zeng, X. G.; Ma, Y.; Tang, Y. G.; Liu, Y. N.; Pan, Y. F. Electrochim. Acta 2015, 157, 211.
[20] Hu, P.; Zhu, T.; Wang, X. P.; Wei, X. J.; Yan, M. Y.; Li, J. T.; Luo, W.; Yang, W.; Zhang, W. C.; Zhou, L.; Zhou, Z. Q.; Mai, L. Q. Nano Lett. 2018, 18, 1758.
[21] Avansi, W.; Maia, L. J. Q.; Mourão, H. A. J. L.; Ribeiro, C. J. Alloys Compd. 2018, 731, 1119.
[22] Cao, L. F.; Chen, L.; Huang, Z.; Kuang, Y.; Zhou, H.; Chen, Z. ChemElectroChem 2016, 3, 122.
[23] Dong, Y. F.; Li, S.; Zhao, K. N.; Han, C. H.; Chen, W.; Wang, B. L.; Wang, L.; Xu, B.; Wei, Q. L.; Zhang, L.; Xu, X.; Mai, L. Q. Energy Environ. Sci. 2015, 8, 1267.
[24] Yuan, S.; Liu, Y. B.; Xu, D.; Ma, D. L.; Wang, S.; Yang, X. H.; Cao, Z. Y.; Zhang, X. B. Adv. Sci. 2015, 2, 1400018.
[25] Cai, Y. S.; Liu, F.; Luo, Z. G.; Fang, G. Z.; Zhou, J.; Pan, A. Q.; Liang, S. Q. Energy Storage Materials 2018, 13, 168.
[26] Takeda, T.; Taniki, R.; Masuda, A.; Honma, I.; Akutagawa, T. J. Power Sources 2016, 328, 228.
[27] Radwan, A.; Liu, Y. L.; Qi, Y. Y.; Jin, W.; Nguyen, V. T.; Yang, X.; Yang, S.; Chen, W. Mater. Res. Bull. 2018, 97, 24.
[28] Zhao, Y.; Wang, L. P.; Sougrati, M. T.; Feng, Z. X.; Leconte, Y.; Fisher, A.; Srinivasan, M.; Xu, Z. C. Adv. Energy Mater. 2017, 7, 1601424.
[29] Wang, F. X.; Wu, X. W.; Li, C. Y.; Zhu, Y. S.; Fu, L. Y.; Wu, Y. P.; Liu, X. Energy Environ. Sci. 2016, 9, 3570.
[30] Wan, F.; Zhang, L. L.; Dai, X.; Wang, X. Y.; Niu, Z. Q.; Chen, J. Nat. Commun. 2018, 9, 1656.
[31] Li, F.; Zhou, Z. Small 2018, 14, 1702961.
[32] Wang, L.; Sun, Y. G.; Hu, L. L.; Piao, J. Y.; Guo, J.; Manthiram, A.; Ma, J.; Cao, A. M. J. Mater. Chem. A 2017, 5, 8752.
[33] Guo, X.; Fang, G. Z.; Zhang, W. Y.; Zhou, J.; Shan, L. T.; Wang, L. B.; Wang, C.; Lin, T. Q.; Tang, Y.; Liang, S. Q. Adv. Energy Mater. 2018, 8, 1801819.
[34] Xiao, F.; Song, X.; Li, Z.; Zhang, H.; Zhang, L.; Lei, G.; Xiao, Q.; Hu, Z.; Ding, Y. J. Mater. Chem. A 2017, 5, 17432.
[35] Liu, L.; Qi, X.; Hu, Y.; Chen, L.; Huang, X. Acta Chim. Sinica 2017, 75, 218(in Chinese). (刘丽露, 戚兴国, 胡勇胜, 陈立泉, 黄学杰, 化学学报, 2017, 75, 218.)
[36] Rozier, P.; Galy, J. J. Solid State Chem. 1997, 134, 294.
[37] Hartung, S.; Bucher, N.; Franklin, J. B.; Wise, A. M.; Lim, L. Y.; Chen, H.-Y.; Weker, J. N.; Michel-Beyerle, M.-E.; Toney, M. F.; Srinivasan, M. Adv. Energy Mater. 2016, 6, 1502336.
[38] Kumagai, N.; Yu, A.; West, K. J. Appl. Electrochem. 1997, 27, 953.
[39] Song, H. Q.; Liu, C. F.; Zhang, C. K.; Cao, G. Z. Nano Energy 2016, 22, 1.
[40] Uchaker, E.; Jin, H. G.; Yi, P.; Cao, G. Z. Chem. Mater. 2015, 27, 7082.
[41] Song, X.; Li, J.; Li, Z.; Xiao, Q.; Lei, G.; Hu, Z.; Ding, Y.; Sari, H. M. K.; Li, X. ACS Appl. Mater. Interfaces 2019, 11, 10631.
[42] Peng, C.; Xiao, F.; Yang, J.; Li, Z.; Lei, G.; Xiao, Q.; Ding, Y.; Hu, Z. Electrochim. Acta 2016, 192, 216.
[43] He, H.; Jin, G.; Wang, H.; Huang, X.; Chen, Z.; Sun, D.; Tang, Y. J. Mater. Chem. A 2014, 2, 3563.
[44] Ko, Y. W.; Teh, P. F.; Pramana, S. S.; Wong, C. L.; Su, T.; Li, L.; Madhavi, S. ChemElectroChem 2015, 2, 837.
[45] Haynes, W. M. CRC-Handbook-of-Chemistry-and-Physics (97th Edition), 2016~2017, CRC Press.
[46] Yao, X.; Zhao, Y.; Castro, F. A.; Mai, L. ACS Energy Lett. 2019, 4, 771.
[47] Meng, J.; Liu, Z.; Niu, C.; Xu, X.; Liu, X.; Zhang, G.; Wang, X.; Huang, M.; Yu, Y.; Mai, L. J. Mater. Chem. A 2016, 4, 4893.
[48] Thamodaran, P.; Kesavan, T.; Vivekanantha, M.; Senthilkumar, B.; Barpanda, P.; Sasidharan, M. ACS Appl. Energy Mater. 2019, 2, 852.
[49] Zhou, Y. N.; Ma, J.; Hu, E.; Yu, X.; Gu, L.; Nam, K. W.; Chen, L.; Wang, Z.; Yang, X. Q. Nat. Commun. 2014, 5, 5381.
[50] Wei, Q. L.; Jiang, Z. Y.; Tan, S. S.; Li, Q. D.; Huang, L.; Yan, M. Y.; Zhou, L.; An, Q. Y.; Mai, L. Q. ACS Appl. Mater. Interfaces 2015, 7, 18211.
[51] Clites, M.; Pomerantseva, E. Energy Storage Mater. 2018, 11, 30.
[52] Duan, J.; Zhu, C.; Du, Y.; Wu, Y.; Chen, Z. J. Mater. Sci. 2017, 52, 10470.
/
| 〈 |
|
〉 |