

Lewis Base-Boryl Radical Enabled Giese Reaction and Barton Decarboxylation of N-Hydroxyphthalimide (NHPI) Esters
Received date: 2019-05-13
Online published: 2019-07-09
Supported by
Project supported by the National Natural Science Foundation of China(21672195);Project supported by the National Natural Science Foundation of China(21702201);the Fundamental Research Funds for the Central Universities
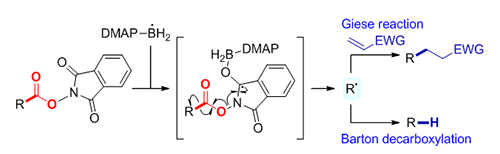
Decarboxylation of N-hydroxyphthalimide (NHPI) esters represents a powerful tool to generate carbon radicals, which has wide applications in the construction of C—C bonds and C—X bonds. Traditionally, the radical decarboxylation of NHPI esters has been enabled by transition-metal catalysis and photoredox catalysis. Recently, several visible light-mediated photosensor-free decarboxylation reactions have been reported with the use of special electron-donor systems. While notable, it’s still highly desirable to develop transition-metal-free, mild, and general methods to realize the radical decarboxylation of NHPI esters. Herein, we report 4-dimethylaminopyridine (DMAP)-boryl radical enabled Giese reaction and Barton decarboxylation of NHPI esters. The reaction starts from the generation of DMAP-boryl radical in the presence of a radical initiator, which then adds specifically to the carbonyl oxygen atom of NHPI ester 2, followed by β-fragmentation to give a nucleophilic carbon radical intermediate. Addition of the carbon radical to electron-deficient alkenes affords the Giese reaction product 4. On the other hand, hydrogen atom transfer from thiol to the nucleophilic carbon radical results in the Barton decarboxylation products 5. The reactions exhibit a broad substrate scope and excellent functional group tolerance. NHPI esters of primary, secondary, and tertiary alkyl carboxylic acids, including bio-active natural products and drugs, proceed smoothly to give the corresponding products in moderate to good yields. A variety of electron-deficient alkenes, such as vinyl esters, vinyl amides and vinyl sulphones, can be used as the Michael acceptors. A general procedure for the Giese reaction is as following: a solution of NHPI ester 2 (0.5 mmol), 4-dimethylaminopyridine-borane (0.6 mmol), AIBN (0.1 mmol) and electron- deficient alkenes 3 (0.4 mmol) in toluene (4.0 mL) was stirred at 80 ℃ for 4 h under nitrogen atmosphere. After evaporation of solvent, the crude residue was purified by flash column chromatography on silica gel (petroleum ether/ethyl acetate) to afford Giese reaction product 4. A general procedure for the Barton decarboxylation is as following: a solution of NHPI ester 2 (0.5 mmol), 4-dimethylaminopyridine-borane (0.55 mmol), TBHN (0.1 mmol) and PhSH (0.1 mmol) in benzotrifluoride (5.0 mL) was stirred at 80 ℃ for 1 h under nitrogen atmosphere. After evaporation of solvent, the crude residue was purified by flash column chromatography on silica gel (petroleum ether/ethyl acetate) to afford decarboxylative reduction product 5.
Jikang Jin, , Fenglian Zhang, , Yifeng Wang, . Lewis Base-Boryl Radical Enabled Giese Reaction and Barton Decarboxylation of N-Hydroxyphthalimide (NHPI) Esters[J]. Acta Chimica Sinica, 2019 , 77(9) : 889 -894 . DOI: 10.6023/A19050173
| [1] | Liu, X.; Wang, Z.; Cheng, X.; Li, C. J. Am. Chem. Soc. 2012, 134, 14330. |
| [2] | Wang, Z.; Zhu, L.; Yin, F.; Su, Z.; Li, Z.; Li, C. J. Am. Chem. Soc. 2012, 134, 4258. |
| [3] | Yin, F.; Wang, Z.; Li, Z.; Li, C. J. Am. Chem. Soc. 2012, 134, 10401. |
| [4] | Liu, C.; Wang, X.; Li, Z.; Cui, L.; Li, C. J. Am. Chem. Soc. 2015, 137, 9820. |
| [5] | Cui, L.; Chen, H.; Liu, C.; Li, C . Org. Lett. 2016, 18, 2188. |
| [6] | Tan, X.; Liu, Z.; Shen, H.; Zhang, P.; Zhang, Z.; Li, C. J. Am. Chem. Soc. 2017, 139, 12430. |
| [7] | Dong, Y.; Wang, Z.; Li, C . Nat. Commun. 2017, 8, 277. |
| [8] | Tan, X.; Song, T.; Wang, Z.; Chen, H.; Cui, L.; Li, C. Org . Lett. 2017, 19, 1634. |
| [9] | Zuo, Z.; MacMillan, D. W. C. J. Am. Chem. Soc. 2014, 136, 5257. |
| [10] | Johnston, C. P.; Smith, R. T.; Allmendinger, S.; MacMillan, D. W. C .Nature 2016, 536, 322. |
| [11] | Bloom, S.; Liu, C.; K?lmel, D. K.; Qiao, J. X.; Zhang, Y.; Poss, M. A.; Ewing, W. R.; MacMillan, D. W. C. Nat. Chem. 2017, 10, 205. |
| [12] | Kautzky, J. A.; Wang, T.; Evans, R. W.; MacMillan, D. W. C. J. Am. Chem. Soc. 2018, 140, 6522. |
| [13] | Liang, Y.; Zhang, X.; MacMillan, D. W. C .Nature 2018, 559, 83. |
| [14] | Le Vaillant, F.; Courant, T.; Waser, J. Angew. Chem., Int. Ed. 2015, 54, 11200. |
| [15] | Zhou, Q.-Q.; Guo, W.; Ding, W.; Wu, X.; Chen, X.; Lu, L.-Q.; Xiao, W.-J. Angew. Chem., Int. Ed. 2015, 54, 11196. |
| [16] | Okada, K.; Okamoto, K.; Oda, M. J. Am. Chem. Soc. 1988, 110, 8736. |
| [17] | Okada, K.; Okamoto, K.; Morita, N.; Okubo, K.; Oda, M. J. Am. Chem. Soc. 1991, 113, 9401. |
| [18] | Cornella, J.; Edwards, J. T.; Qin, T.; Kawamura, S.; Wang, J.; Pan, C.-M.; Gianatassio, R.; Schmidt, M.; Eastgate, M. D.; Baran, P. S . J. Am. Chem. Soc. 2016, 138 2174. |
| [19] | Xuan, J.; Zhang, Z.-G.; Xiao, W.-J. Angew. Chem., Int. Ed. 2015, 54, 15632. |
| [20] | Huang, H.; Jia, K.; Chen, Y . ACS Catal. 2016, 6, 4983. |
| [21] | Jin, Y.; Fu, H. Asian J. Org. Chem. 2017, 6, 368. |
| [22] | Li, Y.; Ge, L.; Muhammad, M. T.; Bao, H . Synthesis 2017, 49, 5263. |
| [23] | Malins, L. R . Pept. Sci. 2018, 110, 24049. |
| [24] | Qin, T.; Cornella, J.; Li, C.; Malins, L. R.; Edwards, J. T.; Kawamura, S.; Maxwell, B. D.; Eastgate, M. D.; Baran, P. S . Science 2016, 352, 801. |
| [25] | Toriyama, F.; Cornella, J.; Wimmer, L.; Chen, T.-G.; Dixon, D. D.; Creech, G.; Baran, P. S. J. Am. Chem. Soc. 2016, 138, 11132. |
| [26] | Wang, J.; Qin, T.; Chen, T.-G.; Wimmer, L.; Edwards, J. T.; Cornella, J.; Vokits, B.; Shaw, S. A.; Baran, P. S. Angew. Chem., Int. Ed. 2016, 55, 9676. |
| [27] | Li, C.; Wang, J.; Barton, L. M.; Yu, S.; Tian, M.; Peters, D. S.; Kumar, M.; Yu, A. W.; Johnson, K. A.; Chatterjee, A. K.; Yan, M.; Baran, P. S . Science 2017, 356, 7355. |
| [28] | Qin, T.; Malins, L. R.; Edwards, J. T.; Merchant, R. R.; Novak, A. J. E.; Zhong, J. Z.; Mills, R. B.; Yan, M.; Yuan, C.; Eastgate, M. D.; Baran, P. S. Angew. Chem., Int. Ed. 2017, 56, 260. |
| [29] | Huihui, K. M. M.; Caputo, J. A.; Melchor, Z.; Olivares, A. M.; Spiewak, A. M.; Johnson, K. A.; DiBenedetto, T. A.; Kim, S.; Ackerman, L. K. G.; Weix, D. J. J. Am. Chem. Soc. 2016, 138, 5016. |
| [30] | Huang, L.; Olivares, A. M.; Weix, D. J. Angew. Chem., Int. Ed. 2017, 56, 11901. |
| [31] | Lackner, G. L.; Quasdorf, K. W.; Overman, L. E. J. Am. Chem. Soc. 2013, 135, 15342. |
| [32] | Lackner, G. L.; Quasdorf, K. W.; Pratsch, G.; Overman, L. E. J. Org. Chem. 2015, 80, 6012. |
| [33] | Slutskyy, Y.; Overman, L. E. Org. Lett. 2016, 18, 2564. |
| [34] | Tlahuext-Aca, A.; Garza-Sanchez, R. A.; Glorius, F. Angew. Chem., Int. Ed. 2017, 56, 3708. |
| [35] | Kachkovskyi, G.; Faderl, C.; Reiser, O. Adv. Synth. Catal. 2013, 355, 2240. |
| [36] | Jiang, M.; Yang, H.; Fu, H . Org. Lett. 2016, 18, 1968. |
| [37] | Jin, Y.; Jiang, M.; Wang, H.; Fu, H . Sci. Rep. 2016, 6, 20068. |
| [38] | Candish, L.; Teders, M.; Glorius, F. J. Am. Chem. Soc. 2017, 139, 7440. |
| [39] | Fawcett, A.; Pradeilles, J.; Wang, Y.; Mutsuga, T.; Myers, E. L.; Aggarwal, V. K . Science 2017, 357, 283. |
| [40] | Fu, M.-C.; Shang, R.; Zhao, B.; Wang, B.; Fu, Y . Science 2019, 363, 1429. |
| [41] | Gao, L.; Wang, G.; Cao, J.; Yuan, D.; Xu, C.; Guo, X.; Li, S. Chem . Commun. 2018, 54, 11534. |
| [42] | Yang, J.; Li, Z.; Zhu,, S . Chin. J. Org. Chem. 2017, 37, 2481. |
| [42] | ( 杨吉民, 李子奇, 朱守非 , 有机化学, 2017, 37, 2481.) |
| [43] | Ren, S.-C.; Zhang, F.-L.; Qi, J.; Huang, Y.-S.; Xu, A.-Q.; Yan, H.-Y.; Wang, Y.-F. J. Am. Chem. Soc. 2017, 139, 6050. |
| [44] | Yu, Y.-J.; Zhang, F.-L.; Cheng, J.; Hei, J.-H.; Deng, W.-T.; Wang, Y.-F. . Org. Lett. 2018, 20, 24. |
| [45] | Jin, J.-K.; Zhang, F.-L.; Zhao, Q.; Lu, J.-A.; Wang, Y.-F. . Org Lett. 2018, 20, 7558. |
| [46] | Qi, J.; Zhang, F.-L.; Huang, Y.-S.; Xu, A.-Q.; Ren, S.-C.; Yi, Z.-Y.; Wang, Y.-F. . Org. Lett. 2018, 20, 2360. |
| [47] | Ren, S.-C.; Zhang, F.-L.; Xu, A.-Q.; Yang, Y.; Zheng, M.; Zhou, X.; Fu, Y.; Wang, Y.-F . Nat. Commun. 2019, 10, 1934. |
| [48] | Franz, J. A.; Bushaw, B. A.; Alnajjar, M. S. J. Am. Chem. Soc. 1989, 111, 268. |
| [49] | Newcomb, M.; Choi, S.-Y.; Horner, J. H. J. Org. Chem. 1999, 64, 1225. |
| [50] | Crich, D.; Grant, D.; Krishnamurthy, V.; Patel, M. Acc. Chem. Res. 2007, 40, 453. |
| [51] | Pan, X.; Lac?te, E.; Lalevée, J.; Curran, D. P. J. Am. Chem. Soc. 2012, 134, 5669. |
| [52] | Dénès, F.; Pichowicz, M.; Povie, G.; Renaud, P. Chem. Rev. 2014, 114, 2587. |
/
| 〈 |
|
〉 |