

Radical Promoted Annulation of Alkynones for the Construction of 2,3-Disubstituted Thiochromones
Received date: 2019-05-11
Online published: 2019-08-12
Supported by
Project supported by the National Natural Science Foundation of China(No. 21602065)
Thiochromones are prevalent structures in various biological active molecules, natural products and potent drug candidates. However, only few methods for the synthesis of thiochromones were reported, and the traditional methods suffer from harsh conditions such as high temperature, strong acid, etc. Recently, synthesis of thiochromones from alkynones had been independently developed by the group of Larock, Mu?ller and Fu. Compared to traditional substances, alkynones are easy to be prepared and handled. More recently, Wu and co-authors improved this synthetic approach via a palladium-catalyzed carbonylative four-component reaction. Despite these great advances, syntheses of diversely functionalized thiochromones, especially 2-functionalized thiochromones which were not easily prepared via the above approaches, are still in demand and highly desirable. As part of our on-going interest in the synthesis of heterocyclic compounds via radical cascade reactions, herein, we developed a radical-involved annulation of methylthiolatedalkynones with diverse radical precursors to access 2-substituted thiochromones. Various substituents such as F, Br and OMe on aromatic ring were all compatible with the reaction, affording the desired 2-substituted thiochromones in moderate to good yields. The most advantage of this protocol is the compatibility of diverse radical precursors including H-phosphorus oxides, aryl aldehydes, arylthiols, BrCF2COOEt, acetone and acetonitrile. Moreover, a series of control experiments were performed to interpret the reaction pathway as a radical process instead of electrophilic cyclization process. Mechanism studies showed that radical involved C(sp 2)—S bond formation and C(sp 3)—S cleavage are the key steps. A general procedure for the radical annulation of alkynones with acetone and acetonitrile is as followed. To the mixture of alkynones 1 (0.2 mmol), in a schlenk flask was added a solution of tert-butyl peroxybenzoate (TBPB) (0.4 mmol) in acetone or acetonitrile (2 mL) under nitrogen atmosphere. The reaction was stirred at 130 or 120 ℃ for 12 h. Upon completion, the reaction mixture was concentrated under vacuum. The residue was purified by silica gel column chromatography using a petroleum ether/ethyl acetate (V:V, 8:1~5:1) to afford the corresponding products 6.
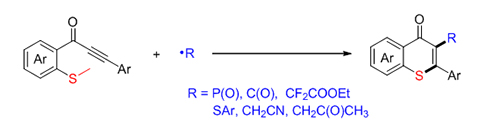
Key words: radical; thiochromen; cyclization reaction; radical cascade reaction
Jian Xu, , Shifan Zhang, , Ying Luo, , Li Zhang, , Fan Zhang, , Tingjing Huang, , Qiuling Song, . Radical Promoted Annulation of Alkynones for the Construction of 2,3-Disubstituted Thiochromones[J]. Acta Chimica Sinica, 2019 , 77(9) : 932 -938 . DOI: 10.6023/A19050169
| [1] | (a) Nakazumi, H.; Ueyama, T.; Kitao, T . J. Heterocycl. Che. 1984, 21, 193. |
| [1] | (b) Couquelet, J.; Tronche, P.; Niviere, P.; Andraud, G . Trav. Soc. Pharm. Montpellie. 1963, 23, 214.. |
| [1] | (c) Nakazumi, H.; Ueyama, T.; Kitao, T . J. Heterocycl. Chem. 1984, 2, 193. |
| [2] | (a) Holshouser, M. H.; Loeffler, L. J.; Hall, I. H . J. Med. Che. 1981, 24, 853. |
| [2] | (b) Razdan, R. K.; Bruni, R. J.; Mehta, A. C.; Weinhardt, K. K.; Papanastassiou, Z. B . J. Med. Che. 1978, 21, 643. |
| [3] | Dhanak, D.; Keenan, R. M.; Burton, G.; Kaura, A.; Darcy, M. G. D.; Shah, H.; Ridgers, L. H.; Breen, A.; Lavery, P.; Tew, D. G.; West, A . Bioorg. Med. Chem. Let. 1998, 8, 3677. |
| [4] | (a) Sangeetha, S.; Sekar, G . Org. Let. 2018, 21, 75. |
| [4] | (b) Zhang, F.; Wu, X. F . J. Org. Che. 2018, 83, 13612. |
| [4] | (c) Kim, H. Y.; Song, E.; Oh, K . Org. Lett. 2017, 19, 312. |
| [4] | (d) Zhu, F.-X.; Wu, X.-F . J. Org. Chem. 2018, 83, 13612. |
| [5] | (a) Schneller, S. W . Adv. Heterocycl. Chem. 1975, 18, 59. |
| [5] | (b) Nakazumi, H.; Wanatabe, S.; Kitaguchi, T.; Kitao, T . Bull. Chem. Soc. Jpn. 1990, 63, 847. |
| [5] | (c) Razdan, R. K.; Bruni, R. J.; Mehta, A. C.; Weinhardt, K. K.; Papanastassiou, Z. B . J. Med. Chem. 1978, 21, 643. |
| [5] | (d) Buggle, K.; Delahunty, J. J.; Philbin, E. M.; Ryan, N. D . J. Chem. Soc. C. 1971, 3168. |
| [6] | Zhou, C.; Dubrovsky, A. V.; Larock, R. C . J. Org. Che. 2006, 71, 1626. |
| [7] | Willy, B.; Frank, W.; Mu?ller, T. J . J. Org. Biomol. Chem. 2010, 8, 90. |
| [8] | Yang, X.-B.; Li, S.-F.; Liu, H.-X.; Jiang, Y.-Y.; Fu, H . RSC Ad. 2012, 2, 6549. |
| [9] | Shen, C.-R.; Spannenberg, A.; Wu, X.-F . Angew. Chem., Int. E. 2016, 55, 5067. |
| [10] | (a) Pan, X.-Q.; Zou, J.-P.; Zhang, G.-L.; Zhang, W . Chem. Commu. 2010, 46, 1721. |
| [10] | (b) Yan, Z.-F.; Xie, J.; Zhu, C.-J . Adv. Synth. Cata. 2017, 359, 4153. |
| [10] | (c) Pan, C.-D.; Huang, B.-F.; Hu, W.-M.; Feng, X.-M.; Yu, J.-T . J. Org. Chem. 2016, 81, 2087. |
| [10] | (d) Zhang, Y.; Ye, S.-Y.; Ji, M.-M.; Li, L.-S.; Guo, D.-M.; Zhu, G.-G . J. Org. Chem. 2017, 82, 6811. |
| [10] | (e) Zhang, Y.; Guo, D.-M.; Ye, S.-Y.; Liu, Z.-C.; Zhu, G.-G . Org. Lett. 2017, 19, 1302. |
| [10] | (f) Zhou, N.-N.; Yang, Z.-F.; Zhang, H.-L.; Wu, Z.-K.; Zhu, C.-J . J. Org. Chem. 2016, 81, 12181. |
| [10] | (g) Zhang, Y.; Zhang, J.-H.; Hu, B.-Y.; Ji, M.-M.; Ye, S.-Y.; Zhu, G.-G . Org. Lett. 2018, 20, 2988. |
| [11] | (a) Hari, D. P.; Hering, T.; Kcning, B . Org. Let. 2012, 14, 5334. |
| [11] | (b) Staples, M. K.; Grange, R. L.; Angus, J. A.; Ziogas, J.; Tan, N. P. H.; Taylor, K. T.; Schiesser, C. H . Org. Biomol. Che. 2011, 9, 473. |
| [11] | (c) Leardini, R.; Pedulli, G. F.; Tundo, A.; Huffman Jr, L. G . Synthesis. 2000, 970. |
| [11] | (d) Zang, H.; Sun, J. G.; Dong, X.; Li, P.; Zhang, B . Adv. Synth. Catal. 2016, 358, 1746. |
| [11] | (e) Yang, W.-C.; Wei, K.; Sun, X.; Zhu, J.; Wu, L . Org. Lett. 2018, 20, 3144. |
| [11] | (f) Xu, J.; Yu, X.-X.; Yan, J.-X.; Song, Q . Org. Lett. 2017, 19, 6292. |
| [11] | (g) Gao, Y.-Z.; Zhang, P.-B.; Li, G.; Zhao, Y.-F . J. Org. Chem. 2018, 83, 13726. |
| [11] | (h) Yan, J.-X.; Xu, J.; Zhou, Y.; Chen, J.; Song, Q . Org. Chem. Front. 2018, 5, 1483. |
| [11] | (i) Liu, W.; Hu, Y.-Q.; Hong, X.-Y.; Li, G.-X.; Huang, X.-B.; Gao, W.-X.; Liu, M.-C.; Xia, Y.; Zhou, Y.-B.; Wu, H.-Y . Chem. Commun. 2018, 54, 14148. |
| [12] | Xu, J.; Zhang, F.; Zhang, S.-F.; Zhang, L.; Yu, X.-X.; Yan, J.-X.; Song, Q . Org. Let. 2019, 21, 1112. |
| [13] | Liu, Q.-Y.; Zhao, X.-H.; Li, J.-L.; Cao, S . Acta Chim. Sinic. 2018, 76, 945. |
| [13] | ( 刘青雲, 赵祥虎, 李佳录, 曹松 , 化学学报 2018, 76, 945.) |
/
| 〈 |
|
〉 |