

Application of Propargylic Radicals in Organic Synthesis
Received date: 2019-06-08
Online published: 2019-08-13
Supported by
Project supported by the National Natural Science Foundation of China(Nos. 21572074);Project supported by the National Natural Science Foundation of China(21772052);Project supported by the National Natural Science Foundation of China(21772053);the Natural Science Foundation of Hubei Province(Nos. 2015CFA033);the Natural Science Foundation of Hubei Province(2017AHB047)
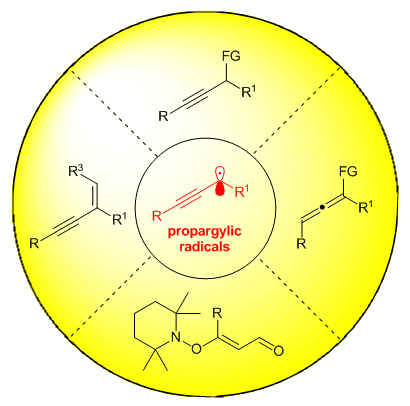
The production and transformation of alkynes occupys an important position in organic synthetic chemistry. Within this realm, propargylic functionalization of alkynes is a feasible way towards this purpose. Especially, the propargylic functionalization via radical pathways has flourished in the last decade, which is believed to be a significant complement to the classic metal-catalyzed propargylation reaction involving cationic intermediates. According to the reaction modes, these advancements will be highlighted by classifying into four types. The first one is the propargylic functionalization reactions involving propargylic radicals. Generally, propargylic radicals can be generated through single electron reduction of alkyne substrates by low-valence metal catalysts or excited state of photocatalysts, then participated in the following cross-coupling reactions to achieve alkyne products. In this part, asymmetric variants have been also well developed. The second one is the preparation of allene compounds through the allenyl radical pathway. For these processes, propargylic radicals can isomerize to allenyl radicals, which can participate in the copper- or nickel-catalyzed coupling reaction to produce significant allene compounds. The third one is the dehydrative alkylation reaction of propargyl alcohols that involve propargylic radical intermediates, too. Such radical intermediates can be further oxidized to propargylic cation intermediates, followed by a deprotonation to form substituted 1,3-enyne compounds. The forth one is the synthesis of vinylic alkoxyamines through a propargylic radical route. Initially, propargyl alcohols can be converted to propargylic radical species by the joint action of copper catalysts and TEMPO. The generated propargylic radical species can be captured by TEMPO to form vinylic alkoxyamines. Finally, an outlook on the radical propargylic functionalizations will be provided at the end of this review.
Fu-Dong Lu, , Xuan Jiang, , Liang-Qiu Lu, , Wen-Jing Xiao, . Application of Propargylic Radicals in Organic Synthesis[J]. Acta Chimica Sinica, 2019 , 77(9) : 803 -813 . DOI: 10.6023/A19060201
| [1] | (a) Trost, B. M.; Li, C.-J. Modern Alkyne Chemistry: Catalytic and Atom-Economic Transformations, Wiley-VCH, New York, 2014. |
| [1] | (b) Trotu?, I. T.; Zimmermann, T.; Schüth, F . Chem. Rev. 2014, 114, 1761. |
| [1] | (c) Tiwari, V. K.; Mishra, B. B.; Mishra, K. B.; Mishra, N.; Singh, A. S.; Chen, X. Chem. Rev. 2016, 116, 3086. |
| [1] | (d) Huang, D.; Liu, Y.; Qin, A.-J.; Tang, B.-Z. Polym. Chem. 2018, 9, 2853. |
| [2] | Ding, C.-H.; Hou, X.-L . Chem. Rev. 2011, 111, 1914. |
| [3] | (a) Nicholas, K. M.; Pettit, R. Tetrahedron Lett. 1971, 37, 3475. |
| [3] | (b) Nicholas, K. M.; Pettit, R. J. Organomet. Chem. 1972, 44, 21. |
| [4] | Melikyan, G. G . Acc. Chem. Res. 2015, 48, 1065. |
| [5] | Geri, R.; Oilizzi, C.; Lardicci, L.; Caporusso, A. M. Gazz. Chim. Ital. 1994, 124, 241. |
| [6] | (a) Miyake, Y.; Uemura, S.; Nishibayashi, Y. ChemCatChem 2009, 1, 342. |
| [6] | (b) Zhang, D.-Y.; Hu, X.-P. Tetrahedron Lett. 2015, 56, 283. |
| [6] | (c) Xiao, Y.-L.; Pan, Q.; Zhang, X.-G. Acta Chim. Sinica 2015, 73, 383 (in Chinese). |
| [6] | (肖玉兰, 潘强, 张新刚, 化学学报, 2015, 73, 383). |
| [7] | Bruneau, C.; Dixneuf, P. H . Metal Vinylidenes and Allenylidenes in Catalysis, Wiley-VCH, Weinheim, 2008. |
| [8] | (a) Kropf, H.; Schr?der, R.; F?lsing, R. Synthesis 1977,894. |
| [8] | (b) Alvarez, L. X.; Christ, M. L.; Sorokin, A. B. Appl. Catal. A: Gen. 2007, 325, 303. |
| [9] | Smith, S. W.; Fu, G. C. J. Am. Chem. Soc. 2008, 130, 12645. |
| [10] | Oelke, A. J.; Sun, J.-W.; Fu, G. C. J. Am. Chem. Soc. 2012, 134, 2966. |
| [11] | Pelphrey, P. M.; Popov, V. M.; Joska, T. M.; Beierlein, J. M.; Bolstad, E. S. D.; Fillingham, Y. A.; Wright, D. L.; Anderson, A. C. J. Med. Chem. 2007, 50, 940. |
| [12] | Schley, N. D.; Fu, G. C. J. Am. Chem. Soc. 2014, 136, 16588. |
| [13] | Domingo-Legarda, P.; Soler-Yanes, R.; Quirós-López, M. T.; Bu?uel, E.; Cárdenas, D. J. Eur. J. Org. Chem. 2018, 35, 4900. |
| [14] | An, L.; Tong, F.-F.; Zhang, X.-G . Acta Chim. Sinica 2018, 76, 977 (in Chinese). |
| [14] | ( 安伦, 童非非, 张新刚, 化学学报, 2018, 76, 977). |
| [15] | Lu, F.-D.; Liu, D.; Zhu, L.; Lu, L.-Q.; Yang, Q.; Zhou, Q.-Q.; Wei, Y.; Lan, Y.; Xiao, W.-J. J. Am. Chem. Soc. 2019, 141, 6167. |
| [16] | Cheng, J.-K.; Loh, T.-P. J. Am. Chem. Soc. 2015, 137, 42. |
| [17] | Andia, A. A.; Miner, M. R.; Woerpel, K. A. Org. Lett. 2015, 17, 2704. |
| [18] | Miner, M. R.; Woerpel, K. A . Eur. J. Org. Chem. 2016,1860. |
| [19] | Cheng, J.-K.; Shen, L.; Wu, L.-H.; Hu, X.-H.; Loh, T.-P . Chem. Commun. 2017, 53, 12830. |
| [20] | (a) Chen, Z.-Y.; Zhou, D.-S.; Zhou, J.-Y.;Wu, S.-H. Acta Chim. Sinica 1997, 55, 1138 (in Chinese). |
| [20] | ( 陈招银, 周大顺, 周景尧, 吴世晖 , 化学学报, 1997, 55, 1138). |
| [20] | (b) Ma, S.-M. Chem. Rev. 2005, 105, 2829. |
| [20] | (c) Lo, V. K.; Wong, M.; Che, C. Org. Lett. 2008, 10, 517. |
| [20] | (d) Yang, L.-J.; Ma, J.-A. Acta Chim. Sinica 2016, 74, 130 (in Chinese) |
| [20] | (杨丽军, 马军安, 化学学报, 2016, 74, 130) |
| [20] | (e) Huang, X.; Ma, S.-M. Acc. Chem. Res. 2019, 52, 1301. |
| [21] | (a) Wartenberg, F.-H.; Junga, H.; Blechert, S . Tetrahedron Lett. 1993, 34, 5251. |
| [21] | (b) Alameda-Angulo, C.; Quiclet-Sire, B.; Zard, S. Z. Tetrahedron Lett. 2006, 47, 913. |
| [22] | Soler-Yanes, R.; Arribas-álvarez, I.; Guisán-Ceinos, M.; Bu?uel, E.; Cárdenas, D. J. Chem. Eur. J. 2017, 23, 1584. |
| [23] | Wang, F.; Wang, D.-H.; Zhou, Y.; Liang, L.; Lu, R.-H.; Chen, P.-H.; Lin, Z.-Y.; Liu, G.-S. Angew. Chem., Int. Ed. 2018, 57, 7140. |
| [24] | Zhu, X.; Deng, W.; Chiou, M.-F.; Ye, C.; Jian, W.; Zeng, Y.; Jiao, Y.; Ge, L.; Li, Y.; Zhang, X.; Bao, H. J. Am. Chem. Soc. 2019, 141, 548. |
| [25] | Ye, C.-Q.; Li, Y.-J.; Zhu, X.-T.; Hu, S.-M.; Yuan, D.-Q.; Bao, H.-L . Chem. Sci. 2019, 10, 3632. |
| [26] | Zhang, K.-F.; Bian, K.-J.; Li, C.; Sheng, J.; Li, Y.; Wang, X.-S. Angew. Chem. Int. Ed. 2019, 58, 5069. |
| [27] | Ye, C.-Q.; Qian, B.; Li, Y.-J.; Su, M.; Li, D.-L.; Bao, H.-L . Org. Lett. 2018, 20, 3202. |
| [28] | Kang, Y.-W.; Choi, Y.-J.; Jang, H.-Y . Org. Lett. 2014, 16, 4842. |
| [29] | Horn, E. J.; Rosen, B. R.; Chen, Y.; Tang, J.; Chen, K.; Eastgate, M. D.; Baran, P. S . Nature 2016, 533, 77. |
/
| 〈 |
|
〉 |