

Pd-Catalyzed Three-Component Chemospecific Allylic Substitution Cascade for the Synthesis of N-Carbonylmethylene-2-Pyridones
Received date: 2019-06-13
Online published: 2019-08-15
Supported by
Project supported by the National Natural Science Foundation of China(21971162);Project supported by the National Natural Science Foundation of China(21672142);Project supported by the National Natural Science Foundation of China(21620102003);Project supported by the National Natural Science Foundation of China(21831005);Shanghai Municipal Education Commission(201701070002E00030)
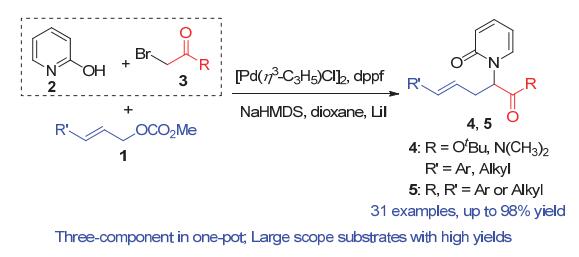
Functionalized N-carbonylmethylene-2-pyridones are some of the most important structural motifs and exist in many natural products and bioactive compounds. Thus, the efficient construction of such skeletons has attracted much attention. Generally, the synthesis of N-carbonylmethylene-2-pyridones is realized via an intermolecular nucleophilic substitution of 2-hydroxypyridines and appropriate electrophiles. However, the above reactions often suffer from low yields caused by poor O/N chemoselectivities due to the dual nucleophilicity of the 2-hydroxypyridines. As far as the structure is concerned, N-carbonylmethylene-2-pyridones can be divided into three sections: a pyridone, a carbonylmethyl group and a side chain. When the side chain is a H atom, the N-substituted pyridones can be constructed conveniently via a reaction of 2-hydroxypyridines and primary α-bromocarbonyl compounds in high yields with excellent chemoselectivities. However, when the side chain is not a H atom, for example an alkyl group, only limited examples have been reported and only moderate yields of the desired N-substituted pyridine products are obtained by a combination of 2-hydroxypyridines and bulky secondary α-bromocarbonyl compounds, mainly due to the poor O/N chemoselectivities. To achieve a general synthetic pathway for the latter, the following practical strategy was designed. 2-Hydroxypyridines were first treated with primary α-bromocarbonyl compounds to generate the unique N-substituted intermediates in situ, which then reacted with the side chain electrophiles to give only the N-alkylated final products. Thus, a Pd-catalyzed three-component chemospecific allylic substitution cascade has been developed for the synthesis of N-carbonylmethylene-2-pyridone derivatives, with the desired products being obtained in up to 98% yield. No O-alkylated by-product was observed. The results suggested that the N-carbonylmethylene-2-pyridones are constructed via a cascade reaction consisting of a nucleophilic substitution followed by an allylic alkylation. The reaction was performed on a gram scale and the corresponding alkylated product was conveniently converted to a pyridone-containing unnatural amino acid. This methodology allows for the highly chemoselective synthesis of biologically important N-carbonylmethylene-2-pyridone derivatives.
Kun Yao, , Hao Liu, , Qianjia Yuan, , Yangang Liu, , Delong Liu, , Wanbin Zhang, . Pd-Catalyzed Three-Component Chemospecific Allylic Substitution Cascade for the Synthesis of N-Carbonylmethylene-2-Pyridones[J]. Acta Chimica Sinica, 2019 , 77(10) : 993 -998 . DOI: 10.6023/A19060210
| [1] | For selected reviews, see: (a) Lenglet, A.; Liabeuf, S.; Bodeau, S.; Louvet, L.; Mary, A.; Boullier, A.; Lemaire-Hurte, A. S.; Jonet, A.; Sonnet, P.; Kamel, S.; Massy, Z. A. Toxins 2016, 8, 339. |
| [1] | (b) Stazi, G.; Zwergel, C.; Mai, A.; Valente, S . Expert Opin. Ther. Pat. 2017, 27, 797. |
| [1] | (c) Fioravanti, R.; Stazi, G.; Zwergel, C.; Valente, S.; Mai, A . Chem. Rec. 2018, 18, 1818. |
| [1] | (d) Shao, T.; Jiang, Z . Acta Chim. Sinica 2017, 75, 70. |
| [1] | ( 邵天举, 江智勇, . 化学学报, 2017, 75, 70.) |
| [1] | (e) Ye, M.; Qiu, S.; Yin, G . Chin. J. Org. Chem. 2017, 37, 667. |
| [1] | ( 叶明琰, 邱少中, 殷国栋, . 有机化学, 2017, 37, 667.) |
| [1] | (c) Bai, F.; Hu, D.; Liu, Y.; Wei, L . Chin. J. Org. Chem. 2018, 38, 2054. |
| [1] | ( 白飞成, 胡德庆, 刘云云, 韦丽, . 有机化学, 2018, 38, 2054.) |
| [2] | For selected reviews, see: (a) Torres, M.; Gil, S.; Parra, M. Curr. Org. Chem. 2005, 9, 1757. |
| [2] | (b) Hill, M. D.; Movassaghi, M. Chem.-Eur. J. 2008, 14, 6836. |
| [2] | For selected examples, see: (c) Fang, Y.-Q.; Bio, M. M.; Hansen, K. B.; Potter, M. S.; Clausen, A . J. Am. Chem. Soc. 2010, 132, 15525. |
| [2] | (d) Li, B.; Wang, G.; Yang, M.; Xu, Z.; Zeng, B.; Wang, H.; Shen, J.; Chen, K.; Zhu, W . Eur. J. Med. Chem. 2013, 70, 677. |
| [2] | (e) Li, C.; K?hny, M.; Breit, B . Angew. Chem., Int. Ed. 2014, 53, 13780. |
| [2] | (f) Zhang, X.; Yang, Z.-P.; Huang, L.; You, S.-L . Angew. Chem., Int. Ed. 2015, 54, 1873. |
| [2] | (g) Feng, B.; Li, Y.; Li, H.; Zhang, X.; Xie, H.; Cao, H.; Yu, L.; Xu, Q . J. Org. Chem. 2018, 83, 6769. |
| [3] | (a) Sato, T.; Yoshimatsu, K.; Otera, J . Synlett 1995,845. |
| [3] | (b) Liu, H.; Ko, S.-B.; Josien, H.; Curran, D. P. Tetrahedron Lett. 1995, 36, 8917. |
| [4] | For selected, examples see: (a) Itami, K.; Yamazaki, D.; Yoshida, J.-I . Org. Lett. 2003, 5, 2161. |
| [4] | (b) Rodrigues, A.; Lee, E. E.; Batey, R. A . Org. Lett. 2010, 12, 260. |
| [4] | (c) Yeung, C. S.; Hsieh, T. H. H.; Dong, V. M . Chem. Sci. 2011, 2, 544. |
| [4] | (d) Tasker, S. Z.; Bosscher, M. A.; Shandro, C. A.; Lanni, E. L.; Ryu, K. A.; Snapper, G. S.; Utter, J. M.; Ellsworth, B. A.; Anderson, C. E . J. Org. Chem. 2012, 77, 8220. |
| [4] | (e) Pan, S.; Ryu, N.; Shibata, T . Org. Lett. 2013, 15, 1902. |
| [4] | (f) Cheng, L.-J.; Brown, A. P. N.; Cordier, C. J . Chem. Sci. 2017, 8, 4299. |
| [5] | (a) Ogata, M.; Matsumoto, H.; Kida, S.; Shimizu, S.; Tawara, K.; Kawamura, Y . J. Med. Chem. 1987, 30, 1497. |
| [5] | (b) Straub, C. S.; Padwa, A . Org. Lett. 1999, 1, 83. |
| [5] | (c) Reichelt, A.; Bur, S. K.; Martin, S. F . Tetrahedron 2002, 58, 6323. |
| [5] | (d) Abreo, M. A.; Meng, J. J.; Agree, C. S . WO 2002016365 2002. |
| [5] | (e) McArdle, B. M.; Quinn, R. J . ChemBioChem 2007, 8, 788. |
| [5] | (f) Jiang, M. X.; Zhou, Y. J . J. Asian Nat. Prod. Res. 2008, 10, 1009. |
| [5] | (g) Payne, R. J.; Bulloch, E. M. M.; Kerbarh, O.; Abell, C . Org. Biomol. Chem. 2010, 8, 3534. |
| [5] | (h) Micale, N.; Ettari, R.; Lavecchia, A.; Di Giovanni, C.; Scarbaci, K.; Troiano, V.; Grasso, S.; Novellino, E.; Schirmeister, T.; Zappalà, M . Eur. J. Med. Chem. 2013, 64, 23. |
| [5] | (i) Scarbaci, K.; Troiano, V.; Micale, N.; Ettari, R.; Tamborini, L.; Di Giovanni, C.; Cerchia, C.; Grasso, S.; Novellino, E.; Schirmeister, T.; Lavecchia, A.; Zappalà, M . Eur. J. Med. Chem. 2014, 76, 1. |
| [6] | For selected examples, see: (a) Bannwarth, L.; Kessler, A.; Pèthe, S.; Collinet, B.; Merabet, N.; Boggetto, N.; Sicsic, S.; Reboud-Ravaux, M.; Ongeri, S . J. Med. Chem. 2006, 49, 4657. |
| [6] | (b) Gibson, S.; Fernando, R.; Jacobs, H. K.; Gopalan, A. S . Tetrahedron 2015, 71, 9271. |
| [6] | (c) Loughlin, W. A.; Jenkins, I. D.; Karis, N. D.; Healy, P. C . Eur. J. Med. Chem. 2017, 127, 341. |
| [6] | (d) Dawson, T. K.; Dziedzic, P.; Robertson, M. J.; Cisneros, J. A.; Krimmer, S. G.; Newton, A. S.; Tirado-Rives, J.; Jorgensen, W. L . ACS Med. Chem. Lett. 2017, 8, 1287. |
| [7] | For selected examples, see: (a) DeRuiter, J.; Brubaker, A. N.; Whitmer, W. L.; Stein, J. L . J. Med. Chem 1986, 29, 2024. |
| [7] | (b) New, J. S.; Christopher, W. L.; Jass, P. A . J. Org. Chem 1989, 54, 990. |
| [7] | (c) , . Eur. J. Org. Chem. 2006, 2715. |
| [7] | (d) Litchfield, J.; Sharma, R.; Atkinson, K.; Filipski, K. J.; Wright, S. W.; Pfefferkorn, J. A.; Tan, B.; Kosa, R. E.; Stevens, B.; Tu, M.; Kalgutkar, A. S . Bioorg. Med. Chem. Lett. 2010, 20, 6262. |
| [7] | (e) Torhan, M. C.; Peet, N. P.; Williams, J. D . Tetrahedron Lett. 2013, 54, 3926. |
| [7] | (f) Xin, B.-T.; de Bruin, G.; Plomp, J.-W.; Florea, B. I.; van der Marel, G. A.; Overkleeft, H. S . Eur. J. Org. Chem. 2016, 1132. |
| [8] | Selected reviews of Pd-catalyzed allylic substitutions: (a) Trost, B. M.; Van Vranken, D. L. Chem. Rev. 1996, 96, 395. |
| [8] | (b) Helmchen, G.; Pfaltz, A. Acc. Chem. Res. 2000, 33, 336. |
| [8] | (c) Trost, B. M.; Crawley, M. L. Chem. Rev. 2003, 103, 2921. |
| [8] | (d) Lu, Z.; Ma, S. Angew. Chem., Int. Ed. 2008, 47, 258. |
| [8] | (e) Trost, B. M.; Zhang, T.; Sieber, J. D . Chem. Sci. 2010, 1, 427. |
| [8] | (f) Tosatti, P.; Nelson, A.; Marsden, S. P . Org. Biomol. Chem. 2012, 10, 3147. |
| [8] | (g) Trost, B. M . Org. Process Res. Dev. 2012, 16, 185. |
| [8] | (h) Lumbroso, A.; Cooke, M. L.; Breit, B . Angew. Chem., Int. Ed. 2013, 52, 1890. |
| [8] | (i) Butt, N. A.; Liu, D.; Zhang, W . Synlett 2014, 25, 615. |
| [8] | (j) Zhuo, C.-X.; Zheng, C.; You, S.-L . Acc. Chem. Res. 2014, 47, 2558. |
| [8] | (k) Butt, N. A.; Zhang, W . Chem. Soc. Rev. 2015, 44, 7929. |
| [8] | (l) Butt, N.; Yang, G.; Zhang, W . Chem. Rec. 2016, 16, 2687. |
| [8] | (m) Fu, J.; Huo, X.; Li, B.; Zhang, W . Org. Biomol. Chem. 2017, 15, 9747. |
| [9] | Selected recent, papers: (a) Zhao, X.; Liu, D.; Guo, H.; Liu, Y.; Zhang, W. J. Am. Chem. Soc. 2011, 133, 19354. |
| [9] | (b) Zhao, X.; Liu, D.; Xie, F.; Liu, Y.; Zhang, W Org. Biomol. Chem 2011, 9, 1871. |
| [9] | (c) Huo, X.; Quan, M.; Yang, G.; Zhao, X.; Liu, D.; Liu, Y.; Zhang, W . Org. Lett 2014, 16, 1570. |
| [9] | (d) Huo, X.; Yang, G.; Liu, D.; Liu, Y.; Gridnev, I. D.; Zhang, W . Angew. Chem., Int. Ed 2014, 53, 6776. |
| [9] | (e) Wei, X.; Liu, D.; An, Q.; Zhang, W . Org. Lett 2015, 17, 5768. |
| [9] | (f) Yao, K.; Liu, D.; Yuan, Q.; Imamoto, T.; Liu, Y.; Zhang, W . Org. Lett 2016, 18, 6296. |
| [9] | (g) An, Q.; Liu, D.; Shen, J.; Liu, Y.; Zhang, W . Org. Lett 2017, 19, 238. |
| [9] | (h) Xia, C.; Shen, J.; Liu, D.; Zhang, W . Org. Lett 2017, 19, 4251. |
| [9] | (i) Huo, X.; He, R.; Fu, J.; Zhang, J.; Yang, G.; Zhang, W . J. Am. Chem. Soc 2017, 139, 9819. |
| [9] | (j) Huo, X.; Fu, J.; He, X.; Chen, J.; Xie, F.; Zhang, W . Chem. Commun 2018, 54, 599. |
| [9] | (k) Yao, K.; Yuan, Q.; Qu, X.; Liu, Y.; Liu, D.; Zhang, W . Chem. Sci 2019, 10, 1767. |
| [9] | We also developed several Ir-catalyzed asymmetric allylic substitution reactions, see: (l) Huo, X.;He, R.;Zhang, X.;Zhang, W . J. Am. Chem. Soc. , 2016, 138, 11093. |
| [9] | (m) He, R.; Liu, P.; Huo, X.; Zhang, W . Org. Lett 2017, 19, 5513. |
| [9] | (n) Huo, X.; Zhang, J.; Fu, J.; He, R.; Zhang, W . J. Am. Chem. Soc. 2018, 140, 2080. |
| [10] | For selected reviews, see: (a) de Graaff, C.; Ruijter, E.; Orru, R. V. A . Chem. Soc. Rev. , 2012, 41, 3969. |
| [10] | (b) , . Med. Chem. Commun. , 2012, 3, 1189. |
| [10] | (c) Eppe, G.;Didier, D.;Marek, I . Chem. Rev. , 2015, 115, 9175. |
| [10] | (d) Vetica, F.;de Figueiredo, R. M.;Orsini, M.;Tofani, D.;Gasperi, T . Synthesis , 2015, 47, 2139. |
/
| 〈 |
|
〉 |