

Visualization of the Electrolyte Migration under Electrochemical Process by ToF-SIMS
Received date: 2019-07-27
Online published: 2019-10-09
Supported by
the National Natural Science Foundation of China(21834001);the National Natural Science Foundation of China(21705046)
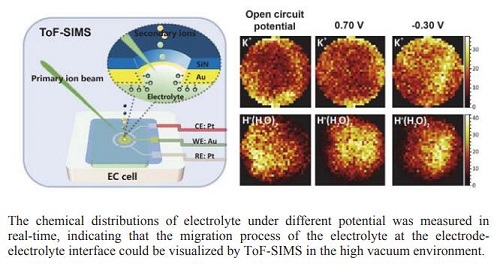
The essence of electrochemical reaction was the electron transfer process on the electrode while the premise for electrochemical reaction happening was the migration and diffusion of electro-active species. Interpreting the monitoring process of electrode towards the electro-active species during the electrochemical reaction would really do favor to the further understanding of the electrochemical evolution process at the electrode-electrolyte interface. In this work, time-of-flight secondary ion mass spectrometry (ToF-SIMS) was adopted for the in-situ monitoring of the electrode-electrolyte interface during the electrochemical reaction with the cooperation of a microfluidic electrochemical cell which was constructed for the liquid sample analysis under the high vacuum environment. With the application of the primary ion beam on the silicon nitride membrane of the microfluidic cell, a micro-hole with the diameter of 2 μm would be fabricated for the direct monitoring of the electrode-electrolyte interface. The migration process of KCl aqueous solution in the confined micropore under the monitoring of gold electrode was investigated by ToF-SIMS here. The direct observation of K+(H2O)n and H+(H2O)n in the electrolyte provided information of the electrode-electrolyte interface at molecular level. Besides, the chemical distributions of K+ and H+(H2O)n under different potential were also studied to verify the feasibility of pore-confined ToF-SIMS in visualizing electrochemical evolution process on the electrode-electrolyte interface. The chemical distributions of K+ and H+(H2O)n obtained by ToF-SIMS showed that K+ would enrich to the surface of gold electrode when the negative potential was applied but diffuse to the bulk solution when positive potential was applied. For H+(H2O)n, they would be repulsed away from the electrode when the positive potential was applied and enrich to the surface of electrode when the negative potential was applied. The potential-dependent behaviors of K+ and H+(H2O)n indicated that visualization of the migration process of electrolyte on the electrode-electrolyte interface was realized, which may help the further study of the evolution at electrode-electrolyte interface during the electrochemical reaction, providing new insight into the revealment of the mechanism of electrochemical reaction.
Hailun Xia , Xin Hua , Yi-Tao Long . Visualization of the Electrolyte Migration under Electrochemical Process by ToF-SIMS[J]. Acta Chimica Sinica, 2019 , 77(11) : 1164 -1167 . DOI: 10.6023/A19070281
| [1] | Sheng W.; Gasteiger H. A.; Shao-Horn Y. J. Electrochem. Soc. 2010, 157, B1529 |
| [2] | Duan X.; Gao Z.; Chang J.; Wu D.; Ma P.; He J.; Xu F.; Gao S.; Jiang K. Electrochim. Acta 2013, 114, 173 |
| [3] | Tanim T. R.; Rahn C. D.; Wang C.-Y. J. Dyn. Syst. Meas. Control. 2015, 137, 11005 |
| [4] | Rosca V.; Beltramo G. L.; Koper M. T. M. J. Electroanal. Chem. 2004, 566, 53 |
| [5] | Ramesha G. K.; Sampath S. J. Phys. Chem. C 2009, 113, 7985 |
| [6] | Zhang J.; Christensen H. E. M.; Ooi B. L.; Ulstrup J. Langmuir 2004, 20, 10200 |
| [7] | Endres F.; Borisenko N.; El Abedin S. Z.; Hayes R.; Atkin R. Faraday Discuss. 2012, 154, 221 |
| [8] | Zeng Z.; Liang W.-I.; Liao H.-G.; Xin H. L.; Chu Y.-H.; Zheng H. Nano Lett. 2014, 14, 1745 |
| [9] | Liu X. H.; Huang J. Y. Energy Environ. Sci. 2011, 4, 3844 |
| [10] | Liu X.; Wang D.; Liu G.; Srinivasan V.; Liu Z.; Hussain Z.; Yang W. Nat. Commun. 2013, 4, 2568 |
| [11] | Wagner M. R.; Albering J. H.; Moeller K.-C.; Besenhard J. O.; Winter M. Electrochem. Commun. 2005, 7, 947 |
| [12] | Lu J.; Hua X.; Long Y.-T. Analyst 2017, 142, 691 |
| [13] | Li H.-W.; Hua X.; Long Y.-T. Anal. Bioanal. Chem. 2019, 411, 4025 |
| [14] | Hua X.; Li H.-W.; Long Y.-T. Anal. Chem. 2017, 90, 1072 |
| [15] | Li H.-W.; Hua X.; Long Y.-T. Chinese J. Anal. Chem. 2018, 46, 61 |
| [15] | 李 好问; 华 鑫; 龙 亿涛 分析化学 2018, 46, 61 |
| [16] | Lee J. C.; Won J.; Chung Y.; Lee H.; Lee E.; Kang D.; Kim C.; Choi J.; Kim J. Appl. Surf. Sci. 2008, 255, 1395 |
| [17] | Liu Y.-Y.; Ying Y.-L.; Hua X.; Long Y.-T. Sci. China Chem. 2018, 61, 159 |
| [17] | 刘 迎亚; 应 佚伦; 华 鑫; 龙 亿涛 中国科学:化学 2018, 61, 159 |
| [18] | Liu B.; Yu X.-Y.; Zhu Z.; Hua X.; Yang L.; Wang Z. Lab Chip 2014, 14, 855 |
| [19] | Hua X.; Xia H.-L.; Long Y.-T. Chem. Sci. 2019, 10, 6215 |
| [20] | Wang J.-G.; Zhang Y.; Yu X.; Hua X.; Wang F.; Long Y.-T.; Zhu Z. J. Phys. Chem. Lett. 2018, 10, 251 |
| [21] | Zhang Y.; Wang J.; Yu X.; Baer D. R.; Zhao Y.; Mao L.; Wang F.; Zhu Z. ACS Energy Lett. 2018, 4, 215 |
| [22] | Wang, Z.; Zhang, Y.; Liu, B.; Wu, K.; Thevuthasan, S.; Baer, D. R.; Zhu, Z.; Yu, X.; Wang, F. 2017, 89, 6. |
| [23] | Tabor R. F.; Morfa A. J.; Grieser F.; Chan D. Y. C.; Dagastine R. R. Langmuir 2011, 27, 6026 |
/
| 〈 |
|
〉 |