

Zn/Ni Bimetallic Relay Catalysis: One Pot Intramolecular Cycloisomerization/Intermolecular Amidoalkylation Reaction toward Oxazole Derivatives
Received date: 2019-08-16
Online published: 2019-10-16
Supported by
the Shandong Provincial Natural Science Foundation(2017YFC1600301);the Youth Science Funds of Shandong Academy of Sciences(2018QN0030);the National Natural Science Foundation of China(51503118)
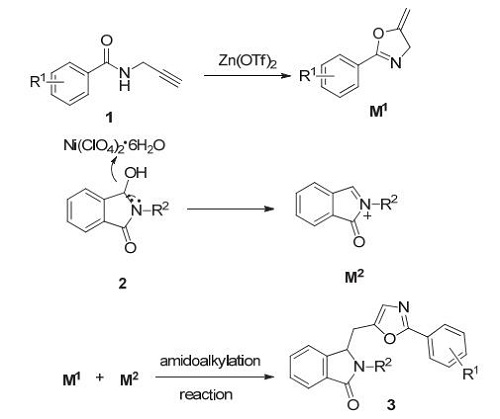
Oxazole derivatives are widely found in natural products and pharmaceuticals with impressive biological properties, tremendous efforts have been devoted to the development of new methodologies and strategies to construct the oxazole rings. However, most of these reactions require harsh reaction conditions, limiting the wide application of these classical oxazole synthetic methods in organic synthesis. N-Acyliminium ions represent important electron deficient carbocations intermediates in organic synthesis because they provide various biologically important natural and unnatural products via C-C and C-heteroatom bondforming methodologies using an inter-or intramolecular path. The removal of a good leaving group at the α-position of amides or lactams usually generates N-acyliminium ions, which act as more electron-deficient carbocations toward nucleophiles. In this paper, a novel tandem metal relay catalytic system of Zn/Ni has been successfully developed. By using this unprecedented Zn(OTf)2/Ni(ClO4)2·6H2O bimetallic relay catalytic system, a variety of oxazole derivatives were obtained from easily available N-(propargyl)-arylamides and various γ-hydroxy lactams through intramolecular cycloisomerization/intermolecular amidoalkylation under mild conditions. The first step of the one-pot procedure is that Zn(OTf)2 acts as a π acid to activate the triple bond of N-(propargyl)-arylamides, and a subsequent intramolecular 5-exo-dig cyclization forms the oxazoline intermediate. Separately, Ni(ClO4)2·6H2O acts as Lewis acid to activate and facilitate the departure of 3-hydroxyl group to form the electrophilic acyliminium ions, which then in an intermolecular reaction is transformed to the oxazole derivatives in good to excellent yield. Control experiments in the optimization section disclose the fact that Zn(OTf)2 and Ni(ClO4)2·6H2O are both indispensable for this intramolecular cycloisomerization/intermolecular amidoalkylation reaction. Generally, the synthetic reactions run under air atmosphere by heating all the substrates and reagents in one-pot at 100℃. The N-(propargyl)-arylamide containing different types of electron-donating substituents, different electron-rich aromatic rings and different electron-withdrawing substituents can react with 3-hydroxy-2-benzyl-isoindolin-1-one to give the corresponding oxazole derivatives. In contrast, the propargyl amide containing an electron withdrawing group has a lower yield than the one using other propargyl amide, because the activity of the oxazoline intermediate obtained by the propargyl amide containing an electron withdrawing group is lower. 3-Hydroxy-2-phenylisoindoline-1-one, 3-hydroxy-2-phenylmethylisoindoline-1-one and 3-hydroxy-2-phenylethylisoindoline-1-one have also been found applicable to this reaction. The present method benefits from the distinctive features of simple reaction conditions, high atom economy and broad substrate tolerance. It is of great significance for the synthesis of oxazole derivatives and the formation of acyliminium ions.
Shuo Zhang , Zitong Hou , Zihe Song , Xiaofeng Su , Feng Wang , Yitao Yu , Dan Peng , Shiqi Cui , Yifan Liu , Jiarui Wang , Jianjun Song . Zn/Ni Bimetallic Relay Catalysis: One Pot Intramolecular Cycloisomerization/Intermolecular Amidoalkylation Reaction toward Oxazole Derivatives[J]. Acta Chimica Sinica, 2019 , 77(11) : 1168 -1172 . DOI: 10.6023/A19080303
| [1] | (a) Wipf, P.; Venkatraman, S. J. Org. Chem. 1996, 61, 6517. |
| [1] | (b) David, N.; Pasceri, R.; Kitson, R. R. A.; Pradal, A.; Moody, C. Chem.-Eur. J. 2016, 22, 10867. |
| [1] | (c) Turchi, I. J.; Dewar, M. J. S. Chem. Rev. 1975, 75, 389. |
| [1] | (d) Meyers, A. I.; Mihelich, E. D. Angew. Chem., Int. Ed. 1976, 15, 270. |
| [1] | (e) Desimoni, G.; Quadrelli, G. F. P. Chem. Rev. 2003, 103, 3119. |
| [1] | (f) Onishi, H. R.; Pelak, B. A.; Gerckens, L. S.; Silver, L. L.; Kahan, F. M.; Chen, M.-H.; Patchett, A. A.; Galloway, S. M.; Hyland, S. A.; Anderson, M. S.; Raetz, C. R. H. Science 1996, 274, 980. |
| [1] | (g) Bergeron, R. J.; Xin, M. G.; Weimar, W. R.; Smith, R. E.; Wiegand, J. J. Med. Chem. 2001, 44, 2469. |
| [1] | (h) Genet, J. P.; Thorimbert, S.; Touzin, A. M. Tetrahedron Lett. 1993, 34, 1159. |
| [1] | (i) Zhou, Q.; Zheng, D. D.; Shi, Y. J.; Yao, W.; Qian, H. W.; Ding, Y.; Wei, Z. H.; Shen, A. B.; Feng, X.; Shi, J.; Dai, H. Chin. J. Org. Chem. 2018, 38, 3318(in Chinese). |
| [1] | (周钱, 郑丹丹, 石玉军, 姚炜, 钱宏炜, 丁颖, 魏中昊, 沈爱宝, 冯霞, 石健, 戴红, 有机化学, 2018, 38, 3318. |
| [1] | (j) Zhou, J. H.; Dai, H.; Qian, H. W.; Du, X. C.; Mao, X. Y.; Shi, Y. J.; Feng, H.; Shi, J.; Yao, Y. Chin. J. Org. Chem. 2018, 38, 2122(in Chinese). |
| [1] | (周家华, 戴红, 钱宏炜, 杜显超, 茅心宇, 石玉军, 冯浩, 石健, 姚勇, 有机化学, 2018, 38, 2122. |
| [1] | (k) Dai, H.; Ding, Y.; Du, X. C.; Yao, W.; Chen, Q. W.; Wang, X. L.; Zhong, S. L.; Cao, X. F.; Shi, Y. J. Chin. J. Org. Chem. 2018, 38, 1755(in Chinese). |
| [1] | (戴红, 丁颖, 杜显超, 姚炜, 陈庆文, 王祥龙, 仲苏林, 曹雄飞, 石玉军, 有机化学, 2018, 38, 1755. |
| [1] | (l) Shi, Y. J.; Du, X. C.; Wang, X. L.; Chen, Q. W.; Li, L.; Dai, H.; Xu, C. Q.; Zhang, J. Y.; Ling, Y. Chin. J. Org. Chem. 2018, 38, 1772(in Chinese). |
| [1] | (石玉军, 杜显超, 王祥龙, 陈庆文, 李玲, 戴红, 徐蔡芹, 张敬远, 凌勇, 有机化学, 2018, 38, 1772.) |
| [2] | (a) Haidukewych, D.; Meyers, A. I. Tetrahedron Lett. 1972, 13, 3031. |
| [2] | (b) Meyers, A. I.; Temple, D. L. J. Am. Chem. Soc. 1970, 92, 6644. |
| [2] | (c) Nelson, T. D.; Meyers, A. I. J. Org. Chem. 1994, 59, 2577. |
| [3] | (a) Lahm, G.; Opatz, T. Org. Lett. 2014, 16, 4201. |
| [3] | (b) Novikau, I.; Hurski, A. Tetrahedron 2018, 74, 1078. |
| [3] | (c) Wang, H. L.; Shang, M.; Sun, S. Z.; Zhou, Z. L.; Laforteza, N.; Dai, H. X.; Yu, J. Q. Org. Lett. 2015, 17, 1228. |
| [3] | (d) Ling, P. X.; Fang, S. L.; Yin, X. S.; Chen, K.; Sun, B. Z.; Shi, B. F. Chem. Eur. J. 2015, 21, 17503. |
| [4] | (a)Desimoni, G.; Faita, G.; Jorgensen, K. A. Chem. Rev. 2006, 106, 3561. |
| [4] | (b) McManus, H. A.; Guiry, P. J. Chem. Rev. 2004, 104, 4151. |
| [4] | (c) Hargaden, G. C.; Guiry, P. J. Chem. Rev. 2009, 109, 2505. |
| [4] | (d) Gade, L. H.; Bellemin-Laponnaz, S. Coord. Chem. Rev. 2007, 251, 718. |
| [5] | (a) Peng, H.; Akhmedov, N. G.; Liang, Y. F.; Jiao, N.; Shi, X. J. Am. Chem. Soc. 2015, 137, 8912. |
| [5] | (b) Hashmi, A. S. K.; Weyrauch, J. P.; Frey, W.; Bats, J. W. Org. Lett. 2004, 6, 4391. |
| [5] | (c) Doherty, S.; Knight, J. G.; Hashmi, A. S. K.; Smyth, C. H.; Ward, N. A. B.; Robson, K. J.; Tweedley, S.; Harrington R. W.; Clegg, W. Organometallics 2010, 29, 4139. |
| [5] | (d) Hashmi, A. S. K.; Schuster, A. M.; Rominger, F. J. Org. Chem. 2012, 77, 6394. |
| [5] | (e) Hashmi, A. S. K.; Schuster, A. M.; Schmuck, M.; Rominger, F. Eur. J. Org. Chem. 2011, 2011, 4595. |
| [6] | (a) Saito, A.; Iimura, K.; Hanzawa, Y. Tetrahedron Lett. 2010, 51, 1471. |
| [6] | (b) Beccalli, E. M.; Borsini, E.; Broggini, G.; Palmisano, G.; Sottocornola, S. J. Org. Chem. 2008, 73, 4746. |
| [6] | (c) Arcadi, A.; Cacchi, S.; Cascia, L.; Fabrizi, G.; Marinelli, F. Org. Lett. 2001, 3, 2501. |
| [7] | (a) Jin, C.; Burgess, J. P.; Kepler, J. A.; Cook, C. E. Org. Lett. 2007, 9, 1887. |
| [7] | (b) Alhalib, A.; Moran, W. J. Org. Biomol. Chem. 2014, 12, 795. |
| [7] | (c) Zhang, S.; Chen, Y.; Wang, J.; Pan, Y.; Xu, Z.; Tung, C.-H. Org. Chem. Front. 2015, 2, 578. |
| [8] | (a) Harmata, M.; Huang, C. Synlett 2008, 1399. |
| [8] | (b) Wong, V. H. L.; White, A. J. P.; Hor, T. S.; Hii, K. K. Adv. Synth. Catal. 2015, 357, 3943. |
| [8] | (c) Hu, Y.; Yi, R.; Wu, F.; Wan, B. J. Org. Chem. 2013, 78, 7714. |
| [9] | (a) Gao, X. H.; Qian, P. C.; Zhang, X. G.; Deng, C. L. Synlett 2016, 27, 1110. |
| [10] | (a) Wang, B.; Chen, Y.; Zhou, L.; Wang, J.; Xu, Z. Org. Biomol. Chem. 2016, 14, 826. |
| [10] | (b) Wang, B.; Chen, Y.; Zhou, L.; Wang, J.; Tung, C.-H.; Xu, Z. J. Org. Chem. 2015, 80, 12718. |
| [11] | (a) Martínez-Estibalez, U.; Gomez-SanJuan, A.; García-Calvo, O.; Aranzamendi, E.; Lete, E.; Sotomayor, N. Eur. J. Org. Chem. 2011, 2011, 3610. |
| [11] | (b) Speckamp, W. N.; Moolenaar, M. J. Tetrahedron 2000, 56, 3817. |
| [11] | (c) Speckamp, W. N.; Hiemstra, H. Tetrahedron 1985, 41, 4367. |
| [11] | (d) Devineau, A.; Pousse, G.; Taillier, C.; Blanchet, J.; Rouden, J.; Dalla, V. Adv. Synth. Catal. 2010, 352, 2881. |
| [11] | (e) Okitsu, O.; Suzuki, R.; Kobayashi, S. J. Org. Chem. 2001, 66, 809. |
| [11] | (f) Raheem, I. T.; Thiara, P. S.; Peterson, E. A.; Jacobsen, E. N. J. Am. Chem. Soc. 2007, 129, 13404. |
| [11] | (g) Muratore, M. C.; Holloway, C. A.; Pilling, A. W.; Storer, R. I.; Trevitt, G.; Dixon, D. J. J. Am. Chem. Soc. 2009, 131, 10796. |
| [12] | (a) Boiaryna, L.; Mkaddem, M. K. E.; Taillier, C.; Dalla, V.; Othman, M. Chem. Eur. J. 2012, 18, 14192. |
| [12] | (b) Yang, T.; Campbell, L.; Dixon, D. J. J. Am. Chem. Soc. 2007, 129, 12070. |
| [13] | Othman R. B.; Affani R.; Tranchant M. J.; Antoniotti S.; Dalla V.; Dunach E. Angew. Chem. Int. Ed. 2010, 49, 776 |
| [14] | Dutta M.; Mandal S. M.; Pegu R.; Pratihar S. J.Org. Chem. 2017, 82, 2193 |
| [15] | (a) Zhang, S.; Wei, F.; Song, C. L.; Jia, J.; Xu, Z. H. Chin. J. Chem. 2014, 32, 937. |
| [15] | (b) Liang, M.; Zhang, S.; Jia, J.; Tung, C.-H.; Wang, J. W.; Xu, Z. H. Org. Lett. 2017, 19, 2526. |
| [15] | (c) Zhang, S.; Xu, Z. L.; Jia, J.; Tung, C.-H.; Xu, Z. H. Chem. Commun. 2014, 50, 12084. |
| [15] | (d) Wang, X. H.; Dong, S. L.; Yao, Z. L.; Feng, L.; Daka, P.; Wang, H.; Xu, Z. H. Org. Lett. 2014, 16, 22. |
/
| 〈 |
|
〉 |