

Palladium-Catalyzed Heck Reaction of Endocyclic Conjugated C=C Bonds of Pyrroles
Received date: 2020-11-14
Online published: 2020-12-15
Supported by
National Natural Science Foundation of China(21772175); National Natural Science Foundation of China(21702184); National Natural Science Foundation of China(22071217); National Natural Science Foundation of China(91956117)
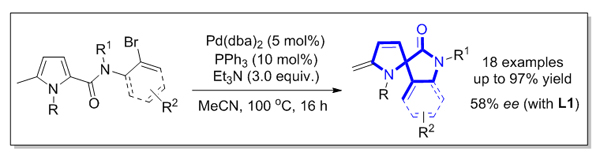
3,2’-Spiropyrrolidine-2-oxindole scaffold is found as a key unit in a number of pharmaceutical candidates and nature products. It is highly desirable to develop efficient synthetic strategies to access such a structural scaffold. Recently, dearomatization reactions have received considerable attention as an efficient and straightforward synthetic method to convert readily available aromatic compounds to three-dimensional organic molecules. Amongst them, the transition-metal-catalyzed dearomative functionalization of endocyclic C=C bonds of aromatic compounds initiated by dearomative migratory insertion has been extensively established. A number of dearomative Heck reactions, reductive Heck reactions, and domino Heck-anionic capture sequences have been developed. Nevertheless, the present studies are mainly focused on the dearomatization reactions of indoles and furans. In contrast, there are rare examples reported for the dearomatization of pyrroles. In this communication, we report a palladium-catalyzed intramolecular Heck reaction of the endocyclic conjugated C=C bonds of C2-tethered pyrroles through an initial migratory insertion to formal conjugate diene and subsequent hydride elimination. A range of 3,2’-spiropyrrolidine-2-oxindole derivatives are obtained in good to excellent yields, showing broad substrate scope. In addition, a preliminary study of enantioselective reaction implies that the target product could be obtained in moderateee under the help of a H8-BINOL-based chiral phosphoramidite ligand. A general procedure for this dearomative Heck reaction is depicted as the follows: to a dried Schlenk tube were added pyrrole substrate 1 (0.20 mmol), Pd(dba)2 catalyst (5.8 mg, 0.010 mmol), and PPh3 ligand (5.2 mg, 0.020 mmol) under N2 atmosphere. 2.0 mL of MeCN solvent and 83 µL of Et 3N were then introduced through a syringe and the Schlenk tube was sealed using a Teflon cap. The resulting reaction mixture was stirred at 100 ℃ for 16 h. After the reaction was completed (monitored by TCL), the mixture was concentrated under vacuum and the residue was purified by flash column chromatography on silica gel to afford the product 2.
Key words: palladium; Heck reaction; pyrrole; oxindole; enantioselectivity
Bo Zhou , Renxiao Liang , Zhongyan Cao , Pinghai Zhou , Yixia Jia . Palladium-Catalyzed Heck Reaction of Endocyclic Conjugated C=C Bonds of Pyrroles[J]. Acta Chimica Sinica, 2021 , 79(2) : 176 -179 . DOI: 10.6023/A20110520
| [1] | (a) Marti C. ; Carreira E.M. Eur. J. Org. Chem. 2003, 2003, 2209. |
| [1] | (b) Trost B.M. ; Brennan M.K. Synthesis 2009, 2009, 3003. |
| [1] | (c) Hong L. ; Wang R. Adv. Synth. Catal. 2013, 355, 1023. |
| [1] | (d) Saraswat P. ; Jeyabalan G. ; Hassan M.Z. ; Rahman M.U. ; Nyola N.K. Synth. Commun. 2016, 46, 1643. |
| [1] | (e) Xu P.-W. ; Yu J.-S. ; Chen C. ; Cao Z.-Y. ; Zhou F. ; Zhou J. ACS Catal. 2019, 9, 1820. |
| [2] | (a) Ali M.A. ; Ismail R. ; Choon T.S. ; Yoon Y.K. ; Wei A.C. ; Pandian S. ; Kumar R.S. ; Osman H. ; Manogaran E. Bioorg. Med. Chem. Lett. 2010, 20, 7064. |
| [2] | (b) George R.F. ; Ismail N.S.M. ; Stawinski J. ; Girgis A.S. Eur. J. Med. Chem. 2013, 68, 339. |
| [2] | (c) Arun Y. ; Bhaskar G. ; Balachandran C. ; Ignacimuthu S. ; Perumal P.T. Bioorg. Med. Chem. Lett. 2013, 23, 1839. |
| [2] | (d) Wu G. ; Ouyang L. ; Liu J. ; Zeng S. ; Huang W. ; Han B. ; Wu F. ; He G. ; Xiang M. Mol. Diversity 2013, 17, 271. |
| [2] | (e) Kumar A. ; Gupta G. ; Bishnoi A.K. ; Saxena R. ; Saini K.S. ; Konwar R. ; Kumar S. ; Dwivedi A. Bioorg. Med. Chem. 2015, 23, 839. |
| [2] | (f) Kathirvelan D. ; Haribabu J. ; Reddy B.S.R. ; Balachandran C. ; Duraipandiyan V. Bioorg. Med. Chem. Lett. 2015, 25, 389. |
| [3] | (a) Pape A.R. ; Kaliappan K.P. ; Ku?ndig E.P. Chem. Rev. 2000, 100, 2917. |
| [3] | (b) López Ortiz, F. ; Iglesias, M.J. ; Fernández, I. ; Andújar Sánchez, C.M.; Gómez, G.R. Chem. Rev. 2007, 107, 1580. |
| [3] | (c) Roche S.P. ; Porco J.A.Jr. Angew. Chem., Int. Ed. 2011, 50, 4068. |
| [3] | (d) Zhuo C.-X. ; Zhang W. ; You S.-L. Angew. Chem., Int. Ed. 2012, 51, 12662. |
| [3] | (e) Zhuo C.-X. ; Zheng C. ; You S.-L. Acc. Chem. Res. 2014, 47, 2558. |
| [3] | (f) Wu W.-T. ; Zhang L. ; You S.-L. Chem. Soc. Rev. 2016, 45, 1570. |
| [3] | (g) Zheng C. ; You S.-L. Chem. 2016, 1, 830. |
| [3] | (h) Wu W.-T. ; Zhang L. ; You S.-L. Acta Chim. Sinica 2017, 75, 419. (in Chinese) |
| [3] | 吴文挺, 张立明, 游书力, 化学学报, 2017, 75, 419. |
| [3] | (i) Wertjes W.C. ; Southgate E.H. ; Sarlah D. Chem. Soc. Rev. 2018, 47, 7996. |
| [3] | (j) Zhu M. ; Zhang X. ; You S.-L. Chem. J. Chin. Univ. 2020, 41, 1407. (in Chinese) |
| [3] | 朱敏, 张霄, 游书力, 高学校化学学报, 2020, 41, 1407. |
| [4] | Zeidan N. ; Lautens M. Synthesis 2019, 51, 4137. |
| [5] | (a) Zhao L. ; Li Z. ; Chang L. ; Xu J. ; Yao H. ; Wu X. Org. Lett. 2012, 14, 2066. |
| [5] | (b) Shen C. ; Liu R.-R. ; Fan R.-J. ; Li Y.-L. ; Xu T.-F. ; Gao J.-R. ; Jia Y.-X. J. Am. Chem. Soc. 2015, 137, 4936. |
| [5] | (c) Petrone D.A. ; Yen A. ; Zeidan N. ; Lautens M. Org. Lett. 2015, 17, 4838. |
| [5] | (d) Petrone D.A. ; Kondo M. ; Zeidan N. ; Lautens M. Chem. Eur. J. 2016, 22, 5684. |
| [5] | (e) Chen S. ; Wu X.-X. ; Wang J. ; Hao X.-H. ; Xia Y. ; Shen Y. ; Jing H. ; Liang Y.-M. Org. Lett. 2016, 18, 4016. |
| [5] | (f) Liu R.-R. ; Xu T.-F. ; Wang Y.-G. ; Xiang B. ; Gao J.-R. ; Jia Y.-X. Chem. Commun. 2016, 52, 13664. |
| [5] | (g) Gao S. ; Yang C. ; Huang Y. ; Zhao L. ; Wu X. ; Yao H. ; Lin A. Org. Biomol. Chem. 2016, 14, 840. |
| [5] | (h) Douki K. ; Ono H. ; Taniguchi T. ; Shimokawa J. ; Kitamura M. ; Fukuyama T. J. Am. Chem. Soc. 2016, 138, 14578. |
| [5] | (i) Wang Y. ; Liu R. ; Gao J. ; Jia Y. Chin. J. Org. Chem. 2017, 37, 691. (in Chinese) |
| [5] | 王永刚, 刘人荣, 高建荣, 贾义霞, 有机化学, 2017, 37, 691. |
| [5] | (j) Qin X. ; Lee M.W.Y. ; Zhou J.S. Angew. Chem., Int. Ed. 2017, 56, 12723. |
| [5] | (k) Liu R.-R. ; Wang Y.-G. ; Li Y.-L. ; Huang B.-B. ; Liang R.-X. ; Jia Y.-X. Angew. Chem., Int. Ed. 2017, 56, 7475. |
| [5] | (l) Xu X. ; Liu J. ; Lu L. ; Wang F. ; Yin B. Chem. Commun. 2017, 53, 7796. |
| [5] | (m) Liu R.-R. ; Xu Y. ; Liang R.-X. ; Xiang B. ; Xie H.-J. ; Gao J.-R. ; Jia Y.-X. Org. Biomol. Chem. 2017, 15, 2711. |
| [5] | (n) Liang R.-X. ; Yang R.-Z. ; Liu R.-R. ; Jia Y.-X. Org. Chem. Front. 2018, 5, 1840. |
| [5] | (o) Zeidan N. ; Beisel T. ; Ross R. ; Lautens M. Org. Lett. 2018, 20, 7332. |
| [5] | (p) Li X. ; Zhou B. ; Yang R.-Z. ; Yang F.-M. ; Liang R.-X. ; Liu R.-R. ; Jia Y.-X. J. Am. Chem. Soc. 2018, 140, 13945. |
| [5] | (q) Wang H. ; Wu X.-F. Org. Lett. 2019, 21, 5264. |
| [5] | (r) Liang R.-X. ; Wang K. ; Wu Q. ; Sheng W.-J. ; Jia Y.-X. Organometallics 2019, 38, 3927. |
| [5] | (s) Shen C. ; Zeidan N. ; Wu Q. ; Breuers C.B.J. ; Liu R.-R. ; Jia Y.-X. ; Lautens M. Chem. Sci. 2019, 10, 3118. |
| [5] | (t) Marchese A.D. ; Lind F. ; Mahon Á. E. ; Yoon H. ; Lautens M . Angew. Chem., Int. Ed. 2019, 58, 5095. |
| [5] | (u) Huang L. ; Cai Y. ; Zhang H.-J. ; Zheng C. ; Dai L.-X. ; You S.-L. CCS Chem. 2019, 1, 106. |
| [5] | (v) Zhang Z. ; Zhang B.-S. ; Li K.-L. ; An Y. ; Liu C. ; Gou X.-Y. ; Liang Y.-M. J. Org. Chem. 2020, 85, 7817. |
| [5] | (w) Yang P. ; Xu R.-Q. ; Zheng C. ; You S.-L. Chin. J. Chem. 2020, 38, 235. |
| [5] | (x) Yang P. Chin. J. Chem. 2020, 38, 525. (y) Zhu, M.; Huang, X.-L.; Xu, H.; Zhang, X.; Zheng, C.; You, S.-L. CCS Chem. 2020, 2, 652. |
| [6] | (a) Liu J. ; Peng H. ; Lu L. ; Xu X. ; Jiang H. ; Yin B. Org. Lett. 2016, 18, 6440. |
| [6] | (b) Liu J. ; Xu X. ; Li J. ; Liu B. ; Jiang H. ; Yin B. Chem. Commun. 2016, 52, 9550. |
| [6] | (c) Liu J. ; Peng H. ; Yang Y. ; Jiang H. ; Yin B. J. Org. Chem. 2016, 81, 9695. |
| [7] | Yao T. ; Zhang F. ; Zhang J. ; Liu L. Org. Lett. 2020, 22, 5063. |
| [8] | (a) Zuo Z. ; Wang H. ; Diao Y. ; Ge Y. ; Liu J. ; Luan X. ACS Catal. 2018, 8, 11029. |
| [8] | (b) Liao X. ; Wang D. ; Huang Y. ; Yang Y. ; You J. Org. Lett. 2019, 21, 1152. |
| [8] | (c) Yang P. ; Zheng C. ; Nie Y.-H. ; You S.-L. Chem. Sci. 2020, 11, 6830. |
| [8] | (d) Zhou B. ; Wang H. ; Cao Z.-Y. ; Zhu J.-W. ; Liang R.-X. ; Hong X. ; Jia Y.-X. Nat. Commun. 2020, 11, 4380. |
| [8] | (e) Chen M. ; Wang X. ; Ren Z.-H. ; Guan Z.-H. CCS Chem. doi.org/10.31635/ccschem.020.202000596. |
| [9] | Huang J. ; Fu R. ; Jing L. ; Qin D. ; Huang K. ; Wang W . Chin. J. Org. Chem. 2019, 39, 456. (in Chinese) |
| [9] | 黄锦, 付荣辉, 敬林海, 秦大斌, 黄昆, 汪伟, 有机化学, 2019, 39, 456.” |
| [10] | (a) Ren H. ; Li Z. ; Knochel P. Chem. - Asian J. 2007, 2, 416. |
| [10] | (b) Yang P. ; You S.-L. Org. Lett. 2018, 20, 7684. |
/
| 〈 |
|
〉 |