

Preparation of α-MnO2 Nanorods/Porous Carbon Cathode for Aqueous Zinc-ion Batteries
Received date: 2020-09-16
Online published: 2020-12-18
Supported by
National Natural Science Foundation of China(51863019); Natural Science Foundation of Gansu Province of China(20JR10RA108); Innovation Fund of Gansu Universities(2020A-013)
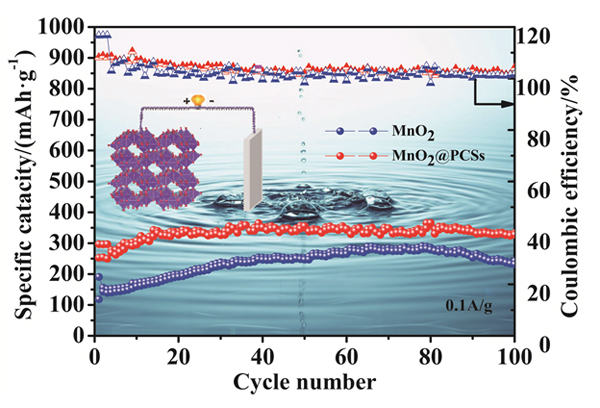
Aqueous zinc-ion batteries (ZIBs) have attracted more attention as large-scale energy storage technology due to their high safety, low cost and environmental benignity. To date, numerous cathodes based on manganese dioxide, vanadium dioxide, and polyanionic compounds have been reported. Among them, Manganese oxides have the advantages of low cost, non-toxicity, abundant materials and high working voltage, have been widely explored as promising cathodes for zinc ion batteries. Among them, MnO2 cathodes are particularly desirable candidates for commercialization owing to their tunnel structure and affordability. α-MnO2has (1×1) and (2×2) tunnel structures, and Zn2+ can rapidly inserted and deserted in the tunnel. However, the capacity of manganese dioxide is fast decaying with the cycles due to the poor conductivity of MnO2, which limits its electrochemical performance. Herein, α-MnO2 nanorods are uniformly distributed on the surface of porous carbon nanosheets network (PCSs) by a simple hydrothermal/dispersion method strategy. In the α-MnO2/PCSs architecture, the α-MnO2 nanorods and α-MnO2/PCSs composite were characterized by X-ray diffraction (XRD), scanning electron microscopy (SEM), high-resolution transmission electron microscopy (HRTEM), etc. The results testified that α-MnO2/PCSs nanorods were firmly adhered on the surface of porous carbon nanosheets, which can effectively avoid the stacking of α-MnO2nanorods, while the high conductive carbon network can improve the electrical conductivity of the composite. The porous network can provide effective electron transfer channels, provide stable hosts for fast Zn2+ extraction/insertion, and prevent the α-MnO2nanorods from stacking each other. Benefiting from the unique porous structure and high conductive network, the α-MnO2/PCSs hybrid shows high reversibility capacity, good rate performance, and outstanding cycling stability. Specifically, α-MnO2/PCSs exhibits high reversible capacity of 350 mAh•g–1 after 100 cycles at 0.1 A•g–1 and maintains a capacity of 160 mAh•g–1 at a high rate of 1 A•g–1 after 1000 cycles, thus making it promising cathode for the high performance ZIBs.
Yanli Li , Dandan Yu , Sen Lin , Dongfei Sun , Ziqiang Lei . Preparation of α-MnO2 Nanorods/Porous Carbon Cathode for Aqueous Zinc-ion Batteries[J]. Acta Chimica Sinica, 2021 , 79(2) : 200 -207 . DOI: 10.6023/A20090428
| [1] | Yabuuchi N.; Kubota K.; Dahbi M.; Komaba S. Chem. Rev. 2014, 114, 11636. |
| [2] | Hu X.F.; Sun J.C.; Li Z.F.; Zhao Q.; Chen C.C.; Chen J. Angew. Chem. Int. Ed. 2016, 128, 6592. |
| [3] | Wang L.; Zhao D.-D.; Liu X.; Yu P.; Fu H.G. Acta Chim. Sinica 2017, 75, 231. (in Chinese) |
| [3] | 王蕾, 赵冬冬, 刘旭, 于鹏, 付宏刚, 化学学报, 2017, 75, 231. |
| [4] | Ren T.; Zhuang Q.C.; Hao Y.W.; Cui Y.L. Acta Chim. Sinica 2016, 74, 833. (in Chinese) |
| [4] | 任彤, 庄全超, 郝玉婉, 崔永丽, 化学学报, 2016, 74, 833. |
| [5] | Zhao Q.; Yan Z.H.; Chen C.C.; Chen J. Chem. Rev. 2017, 117, 10121. |
| [6] | Lee J.; Ju J.B.; Cho W.I.; Cho B.W.; Oh S.H. Electrochim. Acta 2013, 112, 138. |
| [7] | Lee B.; Lee H.R.; Kim H.; Chung K.Y.; Cho B.W.; Oh S.H. Chem. Commun. 2015, 51, 9265. |
| [8] | Häupler B.; Rössel C.; Schwenke A.M.; Winsberg J.; Schmidt D.; Wild A.; Schubert U.S. NPG Asia Mater. 2016, 8, e283. |
| [9] | Xu D.W.; Li B.H.; Wei C.G.; He Y.B.; Du H.D.; Chu X.D.; Qin X.Y.; Yang Q.H.; Kang F.Y. Electrochim. Acta 2014, 133, 254. |
| [10] | Li H.F.; Xu C.J.; Han C.P.; Chen Y.Y.; Wei C.G.; Li B.H.; Kang F.Y. J. Electrochem. Soc. 2015, 162, A1439. |
| [11] | Lee B.; Yoon K.; Seo C.R.; Cho B.W.; Lee H.R.; Yoon C.S.; Oh S.H. ChemSusChem 2016, 9, 2948. |
| [12] | Xu C.J.; Du H.D.; Li B.H.; Kang F.Y.; Zeng Y.Q. Electrochem. Solid-State Lett. 2009, 12, A61. |
| [13] | Pan H.L.; Shao Y.Y.; Yan P.F.; Cheng Y.W.; Han K.S.; Nie Z.M.; Wang C.M.; Yang J.H.; Li X.L.; Bhattacharya P.; Mueller K.T.; Liu J. Nat. Energy 2016, 1, 16039. |
| [14] | Wang J.; Wang J.G.; Liu H.; Wei C.; Kang F. J. Mater. Chem. 2019, 7, 13727. |
| [15] | Alfaruqi M.H.; Mathew V.; Song J.J.; Kim S.; Islam S.; Pham D.T.; Jo J.; Kim S.; Baboo J.P.; Xiu Z.L.; Lee K.S.; Sun Y.K.; Kim J. Chem. Mater. 2017, 29, 1684. |
| [16] | He P.; Yan M.Y.; Zhang G.B.; Sun R.M.; Chen L.N.; An Q.Y.; Mai L.Q. Adv. Energy Mater. 2017, 7, 1601920. |
| [17] | Zeng Y.X.; Zhang X.Y.; Meng Y.; Yu M.H.; Yi J.N.; Wu Y.Q.; Lu X.H.; Tong Y.X. Adv. Mater. 2017, 29, 1700274. |
| [18] | Qiu W.D.; Li Y.; You A.; Zhang Z.M.; Li F.G.; Lu X.H.; Tong Y.X. J. Mater. Chem. 2017, 5, 28. |
| [19] | Alfaruqi M.H.; Gim J.; Kim S.J.; Song J.J.; Jo J.; Kim S.; Mathew V.; Kim J. J. Power Sources 2015, 288, 320. |
| [20] | Alfaruqi M.H.; Mathew V.; Gim J.; Kim S.; Song J.J.; Baboo J.P.; Choi S.H.; Kim J. Chem. Mater. 2015, 27, 3609. |
| [21] | Alfaruqi M.H.; Gim J.; Kim S.; Song J.J.; Pham D.T.; Jo J.; Xiu Z.L.; Mathew V.; Kim J. Electrochem. Commun. 2015, 60, 121. |
| [22] | Fu Y.Q.; Wei Q.L.; Zhang G.X.; Wang X.M.; Zhang J.H.; Hu Y.F.; Wang D.N.; Zuin L.; Zhou T.; Wu Y.C.; Sun S.H. Adv. Energy Mater. 2018, 8, 1801445. |
| [23] | Deng Z.H.; Huang J.D.; Liu J.; Ren L.; Zhu L.Z.; Xiao X.Y.; Tan M.X. Mater. Lett. 2019, 248, 207. |
| [24] | Yan T.T.; Xing G.L.; Ben T. Acta Chim. Sinica 2018, 76, 366. (in Chinese) |
| [24] | 闫婷婷, 邢国龙, 贲腾, 化学学报, 2018, 76, 366. |
| [25] | Tong Z.K.; Fang S.; Zheng H.; Zhang X.G. Acta Chim. Sinica 2016, 74, 185. (in Chinese) |
| [25] | 童震坤, 方姗, 郑浩, 张校刚, 化学学报, 2016, 74, 185. |
| [26] | Yang B.B.; Wang J.; Bin D.; Zhu M.S.; Yang P.; Du Y.K. J. Mater. Chem. 2015, B3, 7440. |
| [27] | Huang Y.; Liu H.; Gong L.; Hou Y.L.; Li Q. J. Power Sources 2017, 347, 29. |
| [28] | Wang D.H.; Li H.F.; Liu Z.X.; Tang Z.J.; Liang G.J.; Mo F.N.; Yang Q.; Ma L.T.; Zhi C.Y. Small 2018, 14, 1803978. |
| [29] | Wang Q.F.; Zou R.Q.; Xia W.; Ma J.; Qiu B.; Mahmood A.; Zhao R.; Yang Y.Y.C.; Xia D.G.; Xu Q. Small 2015, 11, 2511. |
| [30] | Guo S.P.; Li J.C.; Ma Z.; Chi Y.; Xue H.G. J. Mater. Sci. 2016, 52, 2345. |
| [31] | Toupin M.; Brousse T.; Daniel B. Chem. Mater. 2004, 16, 3184. |
| [32] | Wang J.J.; Wang J.G.; Liu H.Y.; Wei C.G.; Kang F.Y. J. Mater. Chem. A 2019, 7, 13727. |
| [33] | Fang G.Z.; Zhou J.; Pan A.Q.; Liang S.Q. ACS Energy Lett. 2018, 3, 2480. |
| [34] | Zhou J.; Shan L.T.; Tang B.Y.; Liang S.Q. Chin. Sci. Bull. 2020, 65, 3562. (in Chinese) |
| [34] | 周江, 单路通, 唐博雅, 梁叔全, 科学通报, 2020, 65, 3562. |
| [35] | Huang J.T.; Zhou J.; Liang S.Q. Acta Phys.-Chim. Sinica 2021, 37, 2005020. (in Chinese) |
| [35] | 黄江涛, 周江, 梁叔全, 物理化学学报, 2021, 37, 2005020. |
| [36] | Huang J.H.; Wang Z.; Hou M.; Dong X.L.; Liu Y.; Wang Y.; Xia Y. Nat. Commun. 2018, 9, 2906. |
| [37] | Xu C.J.; Li B.H.; Du H.D.; Kang F.Y. Angew. Chem. Int. Ed. 2012, 51, 933. |
| [38] | Zuo S.Y.; Xu X.J.; Ji S.M.; Wang Z.S.; Liu Z.B.; Liu J. Chem. Eur. J. 2020, 26, 1. |
| [39] | Wang S.Y.; Wei Q.L.; He P.; Chen Y.; Xu X.; An Q.Y.; Shuang Y.; Shao Y.Y.; Mai L.Q.; Liu J.; Yang J.H. Adv. Mater. 2018, 30, 1703725. |
| [40] | Zhang Y.; Deng S.J.; Luo M.; Pan G.X.; Zeng Y.X.; Lu X.H.; Ai C.Z.; Liu Q.; Xiong Q.Q.; Wang X.L.; Xia X.H.; Tu J.P. Small 2019, 15, 1905452. |
| [41] | Fen X.M.; Huang X.W.; Tan Z.; Zhao B.; Tan S.T. Acta Chim. Sinica 2011, 69, 653. (in Chinese) |
| [41] | 冯小明, 黄先威, 谭卓, 赵斌, 谭松庭, 化学学报, 2011, 69, 653. |
| [42] | Li C.P.; Xie X.S.; Liang S.Q.; Zhou J. Energy Environ. Mater. 2020, 3, 146. |
| [43] | Liu N.N.; Wu X.; Yin Y.Y.; Chen A.S.; Zhao C.Y.; Guo Z.K.; Fan L.S.; Zhang N.Q. ACS Appl. Mater. Interfaces 2020, 12, 28199. |
/
| 〈 |
|
〉 |