

Construction of a Novel Reactive Oxygen Species-responsive Cationic Copolymer and Its Performance in Gene Delivery
Received date: 2021-03-11
Online published: 2021-04-30
Supported by
National Natural Science Foundation of China(21878041); National Natural Science Foundation of China(22078050); Fundamental Research Funds for the Central Universities(DUT17RC(3)059); Fundamental Research Funds for the Central Universities(DUT20YG126); Dalian Science & Technology Innovation Fund(2020JJ26SN050); Dalian Science & Technology Innovation Fund(2020JJ26GX025)
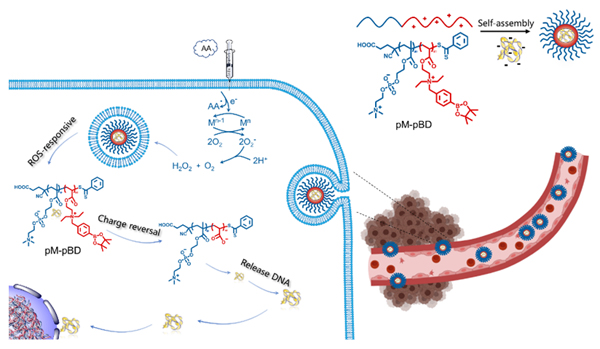
The development of rapid and active gene release function is the key to improve the efficiency of non-viral gene delivery system. Herein, a novel reactive oxygen-responsive cationic block copolymer non-viral gene delivery vector (termed as pM-pBD) consisting of biocompatible poly(2-methacryloyloxyethyl phosphorylcholine) (pMPC) segment and charge reversible poly[(2-acryloyl)-ethyl-(boronic acid benzyl)-diethylammonium bromide] segment (pBD) was synthesized via reversible addition-fragmentation chain transfer polymerization (RAFT). Dynamic light scattering (DLS) assay and ζ potential measurements, transmission electron microscope (TEM) and gel electrophoresis were performed to characterize pM-pBD/(plasmid DNA, pDNA) complexes. Gel electrophoresis retardation assay displayed that pM-pBD can firmly bind pDNA through electrostatic interaction even in the presence of high concentration of heparin, but capable of releasing pDNA in response to reactive oxygen (such as H2O2). The pM-pBD/pDNA complexes show low cytotoxicity against HeLa cells, even at high weight ratio of pM-pBD and pDNA (N/P) (up to 16) demonstrated by MTT (methylthiazolyldiphenyl- tetrazolium bromide) assay. The pM-pBD/pDNA complexes demonstrated appreciable colloidal stability in the presence of 10% fetal bovine serum. The pM-pBD/pDNA complexes at N/P ratio of 3 displayed spherical morphologies with average diameter of approximate 99.1 nm and ζpotential of approximate +13.8 mV. However, once upon incubation in presence of 1 mmol/L H2O2, the diameter of pM-pBD/pDNA complexes at N/P ratio of 3 was enlarged to 330 nm and ζ potential was reversed to –3.91 mV due to charge reversal of BD responsive to reactive oxygen. Flow cytometry revealed uptake efficiency (88.9%) and the highest transfection efficiency (31.2%) for pM-pBD at N/P ratio of 3 and 16, respectively. The transfection efficiency of pM-pBD/pDNA against HeLa cells was observed to be significantly augmented (1.5-fold) after the addition of ascorbic acid, which could stimulate the production of hydrogen peroxide. Therefore, pM-pBD represented intriguing utilities in fabrication of non-viral gene delivery systems, which enable spatiotemporal control of gene release and thereby facilitated the subsequent transcription machinery.
Key words: polycation; charge reversal; gene delivery; ROS-responsive; ascorbic acid
Xu Han , Liuwei Zhang , Qiang Zhang , Xihang Sui , Ming Qian , Qixian Chen , Jingyun Wang . Construction of a Novel Reactive Oxygen Species-responsive Cationic Copolymer and Its Performance in Gene Delivery[J]. Acta Chimica Sinica, 2021 , 79(6) : 794 -802 . DOI: 10.6023/A21030090
| [1] | Shen, Y.; Hu, G. X.; Zhang, H. X.; Qi, L. L.; Luo, C. C. Acta Chim. Sinica 2013, 71, 323. (in Chinese) |
| [1] | (沈银, 胡桂香, 张华星, 齐莉莉, 骆成才, 化学学报, 2013, 71, 323.) |
| [2] | Hirko, A.; Tang, F. X.; Hughes, J. A. Curr. Med. Chem. 2003, 10, 1185. |
| [3] | Nemunaitis, J.; Tong, A. W.; Nemunaitis, M.; Senzer, N.; Phadke, A. P.; Bedell, C.; Adams, N.; Zhang, Y. A.; Maples, P. B.; Chen, S.; Pappen, B.; Burke, J.; Ichimaru, D.; Urata, Y.; Fujiwara, T. Mol. Ther. 2010, 18, 429. |
| [4] | Farmer, Z. L.; Kim, E. S.; Carrizosa, D. R. Oral. Maxil. Surg. Clin. 2018, 31, 117. |
| [5] | Rai, M. F.; Sandell, L. J. J. Am. Acad. Orthop. Sur. 2015, 23, 10. |
| [6] | Wirth, T.; Yla-Herttuala, S. Biomedicines 2014, 2, 2. |
| [7] | Lohr, M. Z.. Gastroenterol. 2006, 44, 4. |
| [8] | Lin, G. M.; Li, L.; Panwar, N.; Wang, J.; Tjin, S. C.; Wang, X. M.; Yong, K. T. Coordin. Chem. Rev. 2018, 374, 133. |
| [9] | Prokofjeva, M. M.; Proshkina, G. M.; Lebedev, T. D.; Shulgin, A. A.; Spirin, P. V.; Prassolov, V. S.; Deyev, S. M. Biochimie. 2017, 142, 226. |
| [10] | Wang, Q.; Zhang, L.; Chen, S. J. China Basic Sci. 2017, 19, 21. (in Chinese) |
| [10] | (王嫱, 张琳, 陈赛娟, 中国基础科学, 2017, 19, 21.) |
| [11] | Li, S. D.; Huang, L. J. Control. Release 2007, 123, 3. |
| [12] | Liu, L.; Yang, J. Y.; Men, K.; He, Z. Y.; Luo, M.; Qian, Z. Y.; Wei, X. W.; Wei, Y. Q. Hum. Gene. Ther. 2018, 29, 110. |
| [13] | Luo, D.; Saltzman, W. M. Nat. Biotechnol. 2000, 18, 33. |
| [14] | Zhou, F.; Wu, C.; Han, F. X.; Zhao, Y. H.; Yuan, X. Y. J. Control. Release 2015, 213, E32. |
| [15] | Srinivas, U. S.; Tan, B. W. Q.; Vellayappan, B. A.; Jeyasekharan, A. D. Redox Biol. 2019, 25, 101084. |
| [16] | Zhao, W. J.; Qiao, Z. Y.; Duan, Z. Y.; Wang, H. Acta Chim. Sinica 2016, 74, 234. (in Chinese) |
| [16] | (赵文静, 乔增莹, 段中余, 王浩, 化学学报, 2016, 74, 234.) |
| [17] | Zhang, L. W.; Chen, Q. X.; Wang, J. Y. Acta Chim. Sinica 2020, 78, 642. (in Chinese) |
| [17] | (张留伟, 陈麒先, 王静云, 化学学报, 2020, 78, 642.) |
| [18] | Stone, J. R.; Yang, S. Antioxid Redox Sign. 2006, 8, 243. |
| [19] | Zhang, J. H.; Wang, W. J.; Zhang, J.; Xiao, Y. P.; Liu, Y. H.; Yu, X. Q. Eur. J. Med. Chem. 2019, 182, 111666. |
| [20] | Shim, M. S.; Xia, Y. N. Angew. Chem. Int. Ed. 2013, 52, 2926. |
| [21] | Chen, Q.; Espey, M. G.; Sun, A. Y.; Pooput, C.; Kirk, K. L.; Krishna, M. C.; Khosh, D. B.; Drisko, J.; Levine, M. Biochemistry 2008, 105, 11105. |
| [22] | Li, J. J.; Ke, W. D.; Wang, L.; Huang, M. M.; Yin, W.; Zhang, P.; Chen, Q. X.; Ge, Z. S. J. Control. Release 2016, 225, 64. |
| [23] | Zhang, L. W.; Qian, M.; Cui, H. Y.; Zeng, S.; Wang, J. Y.; Chen, Q. X. ACS Appl. Mater. Interfaces 2021, 13, 6053. |
| [24] | Wang, J. Y.; Dou, B. R.; Rao, Y. M. Mat. Sci. Eng. C-Mater. 2014, 34, 98. |
/
| 〈 |
|
〉 |