

Fabrication of Polypseudorotaxane-Based Responsive Film via Breath Figure Method
Received date: 2021-03-22
Online published: 2021-05-12
Supported by
National Natural Science Foundation of China(52073092); National Natural Science Foundation of China(51873061); National Natural Science Foundation of China(21404039); Natural Science Foundation of Shanghai(18ZR1408200)
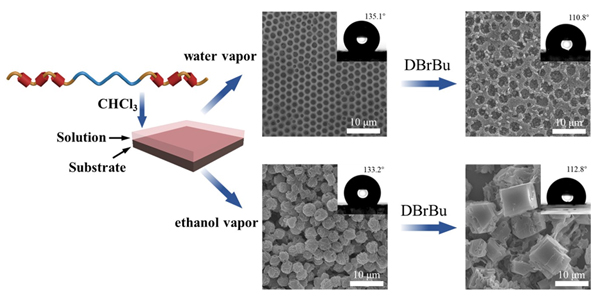
Stimuli-responsive film is an essential part of smart materials for its manipulation of structures or performances in response to external stimulations, which is crucial to the exploitation of actuators, sensors, and soft robotics. Herein, a novel polypseudorotaxane (PPR) with competitive guest-responsiveness was leveraged to fabricate polymer films with diverse surface morphologies via breath figure (BF) method under water or ethanol atmosphere. PPR was constructed based on the selective recognition of 1,4-diethoxypillar[5]arene (DEP5A) to the PCL block of polycaprolactone-block-poly(ethylene glycol)-block-polycaprolactone (PCL-b-PEG-b-PCL) in CHCl3. The morphologies of such polymer films were observed by scanning electron microscopy (SEM). Specifically, ordered honeycomb porous films with an average pore size of approximately 2.15 μm and sphere films with an average diameter of approximately 3.34 μm were fabricated via BF method under water atmosphere and ethanol atmosphere, respectively. The influences of solvent, concentration, and the equivalent ratio of DEP5A to PCL- b-PEG-b-PCL on the nanostructures of films were investigated. It was found that the films with ordered microstructures were obtained using PPR (15.0 equiv. of DEP5A to PCL-b-PEG-b-PCL, 5.0 mg?mL-1 of PCL-b-PEG-b-PCL) solution under a constant relative humidity of 90% or ethanol atmosphere at 25 ℃. These films showed hydrophobic (water contact angle over 130°) due to their hierarchical structures. Furthermore, the obtained films were immersed in competitive guest-containing solution (20.0 μL DBrBu+10.0 mL ethanol) to manipulate their structures and hydrophobicity. The competitive guest, 1,4-dibromobutane (DBrBu), showed stronger affinity than PCL block to DEP5A. DEP5A would be detached from the PCL block and form pseudorotaxane with DBrBu after immersing the films in competitive guest-containing solution. With the immersion time increasing, PPR were destroyed progressively, resulting in the formation of cuboid-crystal films and the decrement of water contact angle. This result provided a facile strategy to fabricate a smart film with controllable wettability and to explore the potential application prospects in the non-destructive transfer of trace liquids, functional coatings and smart film materials.
Jinhui Dong , Jinjie Li , He Wang , Binxiu Liu , Bo Peng , Jianzhuang Chen , Shaoliang Lin . Fabrication of Polypseudorotaxane-Based Responsive Film via Breath Figure Method[J]. Acta Chimica Sinica, 2021 , 79(6) : 803 -808 . DOI: 10.6023/A21030105
| [1] | Zhang, A.; Bai, H.; Li, L. Chem. Rev. 2015, 115, 9801. |
| [2] | Yabu, H.; Jia, R.; Matsuo, Y.; Ijiro, K.; Yamamoto, S.; Nishino, F.; Takaki, T.; Kuwahara, M.; Shimomura, M. Adv. Mater. 2008, 20, 4200. |
| [3] | Lan, P.; Li, J.; Gong, J.; Li, L. Acta Chim. Sinica 2012, 70, 45. (in Chinese) |
| [3] | (兰平, 李剑, 龚剑亮, 李磊, 化学学报, 2012, 70, 45.) |
| [4] | Geng, F.; Chen, J.; Zhao, Q.; Li, J.; Ma, Z. Acta Chim. Sinica 2011, 69, 2741. (in Chinese) |
| [4] | (耿风华, 陈健壮, 赵巧玲, 李剑, 马志, 化学学报, 2011, 69, 2741.) |
| [5] | Yang, X.-Y.; Chen, L.-H.; Li, Y.; Su, B.-L. Chem. Soc. Rev. 2017, 46, 481. |
| [6] | Wu, Y.; Shah, D. U.; Wang, B.; Liu, J.; Ren, X.; Ramage, M. H.; Scherman, O. A. Adv. Mater. 2018, 30, 1707169. |
| [7] | Yang, Y.; Li, X.; Zheng, X.; Chen, Z.; Zhou, Q.; Chen, Y. Adv. Mater. 2018, 30, 1704912. |
| [8] | Xu, Y. Y.; Wang, W.; Chen, J. Z.; Lin, S. L. Chin. J. Org. Chem. 2018, 38, 2161. (in Chinese) |
| [8] | (徐悦莹, 王伟, 陈健壮, 林绍梁, 有机化学, 2018, 38, 2161.) |
| [9] | Heng, L.; Guo, T.; Wang, B.; Fan, L.-Z.; Jiang, L. J. Mater. Chem. A 2015, 3, 23699. |
| [10] | Li, C.; Zhang, Y.; Ju, J.; Cheng, F.; Liu, M.; Jiang, L.; Yu, Y. Adv. Funct. Mater. 2012, 22, 760. |
| [11] | Chen, Y.; Li, F.; Cao, W.; Li, T. J. Mater. Chem. A 2015, 3, 16934. |
| [12] | Ma, J.; Yan, H.; Quan, J.; Bi, J.; Li, H. ACS Appl. Mater. Interfaces 2019, 11, 1665. |
| [13] | Ding, J.; Zhang, A.; Li, L. Soft Matter 2013, 9, 506. |
| [14] | Li, H.; Yang, Y.; Xu, F.; Liang, T.; Wen, H.; Tian, W. Chem. Commun. 2019, 55, 271. |
| [15] | Wang, X.-H.; Song, N.; Hou, W.; Wang, C.-Y.; Wang, Y.; Tang, J.; Yang, Y.-W. Adv. Mater. 2019, 31, 1903962. |
| [16] | Li, X.-S.; Li, Y.-F.; Wu, J.-R.; Lou, X.-Y.; Han, J.; Qin, J.; Yang, Y.-W. J. Mater. Chem. A 2020, 8, 3651. |
| [17] | Chen, J.; Meng, G.; Zhu, Q.; Zhang, S.; Chen, P. J. Mater. Chem. C 2019, 38, 11747. |
| [18] | Catalan, A.; Tiburcio, J. Chem. Commun. 2016, 52, 9526. |
| [19] | Steck, J.; Yang, J.; Suo, Z. ACS Macro Lett. 2019, 8, 754. |
| [20] | Wu, C.-H.; Lu, C.-S.; Chen, W.-L.; Tung, S.-H.; Jeng, R.-J. Macromol. Mater. Eng. 2018, 303, 1700433. |
| [21] | Li, J.; Han, Y.; Chen, C. F. Chin. J. Org. Chem. 2020, 40, 3714. (in Chinese) |
| [21] | (李晶, 韩莹, 陈传峰, 有机化学, 2020, 40, 3714.) |
| [22] | Bertrand, A.; Bousquet, A.; Lartigau-Dagron, C.; Billon, L. Chem. Commun. 2016, 52, 9562. |
| [23] | Wu, X.; Duan, Q. P.; Ni, M. F.; Hu, X. Y.; Wang, L. Y. Chin. J. Org. Chem. 2014, 34, 437. (in Chinese) |
| [23] | (吴旋, 段群鹏, 倪梦飞, 胡晓玉, 王乐勇, 有机化学, 2014, 34, 437.) |
| [24] | Luo, L.; Nie, G.; Tian, D.; Deng, H.; Jiang, L.; Li, H. Angew. Chem. Int. Ed. 2016, 55, 12713. |
| [25] | Tang, X.; Tang, X. Z.; You, Y.; Ren, L.; Wang, Y.; Yan, L. Acta Chim. Sinica 2012, 70, 1565. (in Chinese) |
| [25] | (唐翔, 唐先忠, 游英才, 任立轲, 王洋, 严立京, 化学学报, 2012, 70, 1565.) |
| [26] | Ogoshi, T.; Yoshikoshi, K.; Aoki, T.; Yamagishi, T. Chem. Commun. 2013, 49, 8785. |
| [27] | Fasano, V.; Baroncini, M.; Moffa, M.; Iandolo, D.; Camposeo, A.; Credi, A.; Pisignano, D. J. Am. Chem. Soc. 2014, 136, 14245. |
| [28] | Gao, P.; Wang, P.; Geng, X.; Ye, L.; Zhang, A.; Feng, Z. Acta Chim. Sinica 2013, 71, 347. (in Chinese) |
| [28] | (高鹏, 王培境, 耿雪, 叶霖, 张爱英, 冯增国, 化学学报, 2013, 71, 347.) |
| [29] | Pan, S.; Ni, M.; Mu, B.; Li, Q.; Hu, X.-Y.; Lin, C.; Chen, D.; Wang, L. Adv. Funct. Mater. 2015, 25, 3571. |
| [30] | Ahn, Y.; Jang, Y.; Selvapalam, N.; Yun, G.; Kim, K. Angew. Chem. Int. Ed. 2013, 52, 3140. |
| [31] | Ghasemi, S.; Besharati, M. Polym. Adv. Technol. 2020, 31, 3104. |
| [32] | Li, Y.; Ma, X.; Ma, J.; Zhang, Z.; Niu, Z.; Chen, F. Polymers 2021, 13, 316. |
| [33] | Ma, C.; Zhong, Y.; Li, J.; Chen, C.; Gong, J.; Xie, S.; Li, L.; Ma, Z. Chem. Mater. 2010, 22, 2367. |
| [34] | Du, C.; Zhang, A.; Bai, H.; Li, L. ACS Macro Lett. 2013, 2, 27. |
| [35] | Escalé, P.; Camp, W. V.; Prez, F. D.; Rubatat, L.; Billon, L.; Save, M. Polym. Chem. 2013, 4, 4710. |
| [36] | Zander, N. E.; Orlicki, J. A.; Karikari, A. S.; Long, T. E.; Rawlett, A. M. Chem. Mater. 2007, 19, 6145. |
| [37] | Yabu, H.; Hirai, Y.; Kojima, M.; Shimomura, M. Chem. Mater. 2009, 21, 1787. |
| [38] | Muller, M.; Zentel, R.; Maka, T.; Romanov, S. G.; Torres, C. S. Adv. Mater. 2000, 12, 1499. |
| [39] | Wang, W.; Du, C.; Wang, X.; He, X.; Lin, J.; Li, L.; Lin, S. Angew. Chem. Int. Ed. 2014, 53, 12116. |
| [40] | Wang, W.; Yao, Y.; Luo, T.; Chen, L.; Lin, J.; Li, L.; Lin, S. ACS Appl. Mater. Interfaces 2017, 9, 4223. |
| [41] | Wang, W.; Shen, D.; Li, X.; Yao, Y.; Lin, J.; Wang, A.; Yu, J.; Wang, Z. L.; Hong, S. W.; Lin, Z.; Lin, S. Angew. Chem. Int. Ed. 2018, 57, 2139. |
| [42] | Gao, F.; Yao, Y.; Wang, W.; Wang, X.; Li, L.; Zhuang, Q.; Lin, S. Macromolecules 2018, 51, 2742. |
| [43] | Li, J.; Dong, J.; Cui, K.; Wang, H.; Sun, Y.; Yao, Y.; Chen, J.; Gu, J.; Lin, S. J. Mater. Chem. A 2020, 8, 10917. |
| [44] | Wan, L. S.; Zhu, L. W.; Ou, Y.; Xu, Z. K. Chem. Commun. 2014, 50, 4024. |
/
| 〈 |
|
〉 |