

Direct C—C Bond Formation through Decarboxylative Oxidative Cross-Coupling of Cinnamic Acids with Amides under Metal-Free Condition
Received date: 2021-05-10
Online published: 2021-05-28
Supported by
National Natural Science Foundation of China(21402068); National Natural Science Foundation of China(21502075)
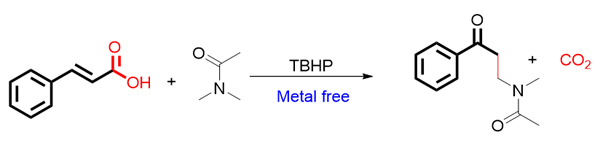
Traditional coupling reactions generally employ metal organic compounds, boron reagents or halogenated compounds as reaction starting materials under the condition of noble metal-catalyst, such as Suzuki reaction and Heck reaction. Although these reactions have been widely used in synthesis, due to the need for prefunctionalization of reactants, they do not have high atomic economy and step economy. Especially for some substrates that are not easy to introduce functional groups, the application of such reactions is limited. In recent years, with the development of organic chemistry, direct C―H bond functionalization has become a research hotspot in organic synthesis. This kind of reaction can overcome the deficiency of traditional coupling reaction, making this kind of reaction more attractive. Amides are very important compounds, which are widely found in natural products and drugs. Through literature investigation, it was found that some functionalization reactions of C(sp3)―H bond adjacent to the nitrogen atom of amides had already reported. Due to the facile synthesis, stability and low toxicity of cinnamic acid compounds, they are also used in organic synthesis to obtain desired product. When we studied the decarboxylation reaction of cinnamic acid compounds, we found that the decarboxylative coupling product of cinnamic acid with amides could be obtained by using amide as solvent, tert-butyl hydroperoxide (TBHP) as oxidant and cinnamic acid as starting material without the use of metal catalyst. The reaction mechanism was also proposed. First, homolysis of tert-butyl alcohol peroxide generated tertiary butyl oxygen free radical and hydroxyl free radicals, the oxidation of C(sp3)―H bonds adjacent to the nitrogen atom of N,N-dimethylacetamide offered carbon free radical, which adds to the α-position of the double bond of cinnamic acid to produce intermediate A. A hydroxyl radical, generated from homolysis of TBHP, then combined with A to generate B, and B was further oxidized to C. Finally, C decarboxylated easily to generate the desired product.
Xiaobiao Lu , Xi Xiao , Changfeng Wan , Zhiyong Wang , Jinbiao Liu . Direct C—C Bond Formation through Decarboxylative Oxidative Cross-Coupling of Cinnamic Acids with Amides under Metal-Free Condition[J]. Acta Chimica Sinica, 2021 , 79(6) : 751 -754 . DOI: 10.6023/A21050200
| [1] | (a) Crabtree, R. H. The Organometallic Chemistry of Transition Metals, 4th ed., Wiley Interscience, New York, 2005, pp. 1~560. |
| [1] | (b) McQuillin, F. J.; Parker, D. G.; Stephenson, G. R. Transition Metal Organometallics for Organic Synthesis, Cambridge University Press, Cambridge, U.K., 1991, pp.1~614. |
| [2] | (a) Saito, B.; Fu, G. C. J. Am. Chem. Soc. 2007, 129, 9602. |
| [2] | (b) Li, S. H.; Lin, Y. J.; Cao, J. G.; Zhang, S. B. J. Org. Chem. 2007, 72, 4067. |
| [2] | (c) Gurung, S. K.; Thapa, S.; Kafle, A.; Dickie, D. A.; Giri, R. Org. Lett. 2014, 16, 1264. |
| [3] | (a) Heck, R. F.; Nolley, J. P. J. Org. Chem. 1972, 37, 2320. |
| [3] | (b) Beletskaya, I. P.; Cheprakov, A. V. Chem. Rev. 2000, 100, 3009. |
| [3] | (c) Cartney, D. M.; Guiry, P. J. Chem. Soc. Rev. 2011, 40, 5122. |
| [4] | (a) Li, C. J. Acc. Chem. Res. 2009, 42, 335. |
| [4] | (b) Xie, Y. X.; Song, R. J.; Xiang, J. N.; Li, J. H. Chin. J. Org. Chem. 2012, 32, 1555. (in Chinese) |
| [4] | (谢叶香, 宋仁杰, 向建南, 李金恒, 有机化学, 2012, 32, 1555.) |
| [4] | (c) Liao, G.; Wu, Y. J.; Shi, B. F. Acta Chim. Sinica 2020, 78, 289. (in Chinese) |
| [4] | (廖港, 吴勇杰, 史炳锋, 化学学报, 2020, 78, 289.) |
| [4] | (d) Zhang, H. H.; Yu, S. Y. Acta Chim. Sinica 2019, 77, 832. (in Chinese) |
| [4] | (张洪浩, 俞寿云, 化学学报, 2019, 77, 832.) |
| [4] | (e) Xiao, L.; Li, J. H.; Wang, T. Acta Chim. Sinica 2019, 77, ?841. (in Chinese) |
| [4] | (肖丽, 李嘉恒, 王挺, 化学学报, 2019, 77, 841.) |
| [4] | (f) Cheng, Z. M.; Chen, P. H.; Liu, G. S. Acta Chim. Sinica 2019, 77,?856. (in Chinese) |
| [4] | (成忠明, 陈品红, 刘国生, 化学学报, 2019, 77, 856.) |
| [4] | (g) Yang, Q. L.; Wang, X. Y.; Weng, X. J.; Yang, X.; Xu, X. T.; Tong, X. F.; Fang, P.; Wu, X. Y.; Mei, T. S. Acta Chim. Sinica 2019, 77, ?866. (in Chinese) |
| [4] | (杨启亮, 王向阳, 翁信军, 杨祥, 徐学涛, 童晓峰, 方萍, 伍新燕, 梅天胜, 化学学报, 2019, 77, 866.) |
| [4] | (h) Xiao, Y. X.; Liu, Z. Q. Acta Chim. Sinica 2019, 77, 874. (in Chinese) |
| [4] | (肖莹霞, 柳忠全, 化学学报, 2019, 77, 874.) |
| [4] | (i) Zhao, Y.; Li, S. H.; Zhang, M. M.; Liu, F. Acta Chim. Sinica 2019, 77, ?916. (in Chinese) |
| [4] | (赵勇, 李施宏, 张苗苗, 刘峰, 化学学报, 2019, 77, 916.) |
| [4] | (j) Wu, Y. J.; Shi, B. F. Chin. J. Org. Chem. 2020, 40,?3517. (in Chinese) |
| [4] | (吴勇杰, 史炳锋, 有机化学, 2020, 40, 3517.) |
| [4] | (k) Yuan, X. Y.; Yang, G. P.; Yu, B. Chin. J. Org. Chem. 2020, 40,?3620. (in Chinese) |
| [4] | (袁晓亚, 杨国平, 於兵, 有机化学, 2020, 40, 3620.) |
| [4] | (l) Jiang, X. L.; Hao, J. Q.; Zhou, G. Q.; Hou, C. C.; Hu, F. D. Chin. J. Org. Chem. 2019, 39,?1811. (in Chinese) |
| [4] | (姜晓蕾, 郝佳奇, 周国庆, 侯程程, 胡芳东, 有机化学, 2019, 39, 1811.) |
| [4] | (m) Li, X. F.; Xiong, W. K.; Ding, Q. P. Chin. J. Org. Chem. 2019, 39, 1867. (in Chinese) |
| [4] | (李小芳, 熊伟康, 丁秋平, 有机化学, 2019, 39, 1867.) |
| [4] | (n) Wang, R. H.; Luan, Y. X.; Ye, M. C. Chin. J. Chem. 2019, 37, 720. |
| [4] | (o) Shi, Z. J.; Wang, L. H.; Cui, X. L. Chin. J. Org. Chem. 2019, 39, 1596. (in Chinese) |
| [4] | (施兆江, 王连会, 崔秀灵, 有机化学, 2019, 39, 1596.) |
| [4] | (p) Shi, B. F.; Zhang, Y. H.; Lam, J. K.; Wang, D. H.; Yu, J. Q. J. Am. Chem. Soc. 2010, 132, 460. |
| [5] | (a) Nieman, J. A.; Coleman, J. E.; Wallace, D. J.; Piers, E.; Lim, L. Y.; Roberge, M.; Andersen, R. J. J. Nat. Prod. 2003, 66, 183. |
| [5] | (b) Loganzo, F.; Discafani, C. M.; Annable, T.; Beyer, C.; Musto, S.; Hari, M.; Tan, X. Z.; Hardy, C.; Hernandez, R.; Baxter, M.; Singanallore, T.; Khafizova, G.; Poruchynsky, M. S.; Fojo, T.; Nieman, J. A.; Ayral-Kaloustian, S.; Zask, A.; Andersen, R. J.; Greenberger, L. M. Cancer Res. 2003, 63, 1838. |
| [6] | (a) Heitz, D. R.; Tellis, J. C.; Molander, G. A. J. Am. Chem. Soc. 2016, 138, 12715. |
| [6] | (b) Kawasaki, T.; Ishida, N.; Murakami, M. J. Am. Chem. Soc. 2020, 142, 3366. |
| [6] | (c) Si, X. J.; Zhang, L. M.; Hashmi, A. S. K. Org. Lett. 2019, 21, 6329. |
| [6] | (d) Fan, X. Z.; Rong, J. W.; Wu, H. L.; Zhou, Q.; Deng, H. P.; Tan, J. D.; Xue, C. W.; Wu, L. Z.; Tao, H. R.; Wu, J. Angew. Chem. Int. Ed. 2018, 57, 8514. |
| [6] | (e) Wang, J.; Li, J.; Huang, J. B.; Zhu, Q. J. Org. Chem. 2016, 81, 3017. |
| [6] | (f) Zhong, R.; Xu, Y.; Sun, M. M.; Wang, Y. R. J. Org. Chem. 2021, 86, 5255. |
| [7] | (a) Tang, R. Y.; Xie, Y. X.; Xie, Y. L.; Xiang, J. N.; Li, J. H. Chem. Commun. 2011, 47, 12867. |
| [7] | (b) Shepherd, N. E.; Tanabe, H.; Xu, Y. J.; Matsunaga, S.; Shibasaki, M. J. Am. Chem. Soc. 2010, 132, 3666. |
| [7] | (c) Mitchell, E. A.; Peschiulli, A.; Lefevre, N.; Meerpoel, L.; Maes, B. U. W. Chem. Eur. J. 2012, 18, 10092. |
| [7] | (d) Dai, C. H.; Meschini, F.; Narayanam, J. M. R.; Stephenson, C. R. J. J. Org. Chem. 2012, 77, 4425. |
| [7] | (e) Li, G. C.; Qian, S. Y.; Wang, C. X.; You, J. S. Angew. Chem. Int. Ed. 2013, 52, 7837. |
| [8] | (a) Zhang, J. X.; Wang, Y. J.; Wang, N. X.; Zhang, W.; Bai, C. B.; Li, Y. H.; Wen, J. L. Synlett 2014,249. |
| [8] | (b) Yan, H.; Lu, L. H.; Rong, G. W.; Liu, D. F.; Zheng, Y.; Chen, J.; Mao, J. C. J. Org. Chem. 2014, 79, 7103. |
| [9] | Ye, W. B.; Yan, Z. C.; Wan, C. F.; Hou, H. Q.; Wang, Z. Y. Acta Chim. Sinica 2018, 76, 99. (in Chinese) |
| [9] | (叶文波, 晏子聪, 万常峰, 侯豪情, 汪志勇, 化学学报, 2018, 76, 99.) |
| [10] | Yang, X. H.; Wei, W. T.; Li, H. B.; Song, R. J.; Li, J. H. Chem. Commun. 2014, 50, 12867. |
/
| 〈 |
|
〉 |