

Effect of Shell Thickness on Skeletal Fe@HZSM-5 Core-Shell Catalysts for Fischer-Tropsch Synthesis
Received date: 2020-12-27
Online published: 2021-05-31
Supported by
National Key Research and Development Project of China(2018YFB0604501); National Natural Science Foundation of China(21872035); International Joint Laboratory on Resource Chemistry (IJLRC) of Shanghai Normal University; Science and Technology Commission of Shanghai Municipality(19DZ2270100)
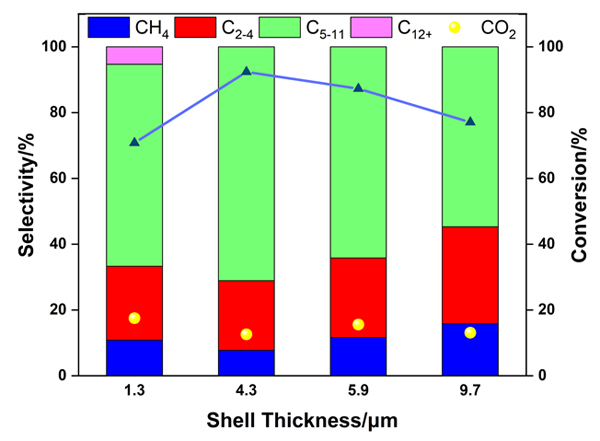
Using the Raney Fe-Al alloy as the precursor for Fe and Al, and tetrapropylammonium hydroxide (TPAOH) as both the base for the leaching of the alloy and the template for the synthesis of HZSM-5, we synthesized the skeletal Fe core-HZSM-5 shell catalysts (Raney Fe@HZSM-5) via a facile one-pot hydrothermal strategy. The thickness of the zeolite shell was adjusted by varying the hydrothermal time. The catalysts were characterized by inductively coupled plasma-atomic emission spectroscopy (ICP-AES), N2 physisorption, X-ray powder diffraction (XRD), temperature-programmed desorption of NH3 (NH3-TPD), and scanning electron microscopy (SEM). It was identified that the Raney Fe-Al alloy was more suitable than the rapidly quenched Fe-Al alloy as the precursor to the skeletal Fe core, since the skeletal Fe catalyst alkali-leached from the former produced more long-chain hydrocarbons during the Fischer-Tropsch synthesis (FTS) reaction for successive cracking and isomerization. A complete HZSM-5 shell was obtained only after above 24 h of hydrothermal treatment. With the hydrothermal time from 2 d to 8 d, the thickness of the compact HZSM-5 shell increased from 1.3 μm to 9.7 μm, the relative crystallinity increased steadily, while the SiO2/Al2O3 ratio remained essentially constant, and the amount of the acid sites is parallel to the thickness of the zeolite shell. In the FTS reaction using syngas with the H2/CO molar ratio of 2 and at 543 K and 2.0 MPa, the CO conversion and the selectivity to the gasoline fraction (C5~C11 hydrocarbons) evolved in a volcanic trend with the shell thickness, suggesting the existence of an optimal amount of the acid sites, while less or more is adverse to the activity and selectivity to the gasoline fraction. The Raney Fe@HZSM-5 catalyst hydrothermally treated for 4 d exhibited the highest CO conversion of 92% and the selectivity to the gasoline fraction of 71%, along with an iso-paraffin to n-paraffin ratio of 1.9. At the n(H2)/n(CO) ratio of 1, the selectivity to the gasoline fraction and the iso-paraffin to n-paraffin ratio were further improved to 73% and 2.1, respectively, which shows promise for this catalyst to transform the coal- or biomass- derived syngas to high-octane number gasoline.
Key words: skeletal Fe; HZSM-5; core-shell structure; Fischer-Tropsch synthesis; gasoline
Dong Sun , Bo Sun , Yan Pei , Shirun Yan , Kangnian Fan , Minghua Qiao , Xiaoxin Zhang , Baoning Zong . Effect of Shell Thickness on Skeletal Fe@HZSM-5 Core-Shell Catalysts for Fischer-Tropsch Synthesis[J]. Acta Chimica Sinica, 2021 , 79(6) : 771 -777 . DOI: 10.6023/A20120563
| [1] | Friedel, R. A.; Anderson, R. B. J. Am. Chem. Soc. 1950, 72, 2307. |
| [2] | Udaya, V.; Rao, S.; Gormley, R. J. Catal. Today 1990, 6, 207. |
| [3] | Dry, M. E. Catalysis Science and Technology, Vol. 1, Eds.: Anderson, J. R.; Boudart, M., Springer, Berlin, 1981,Chapter 4. |
| [4] | Schulz, H.; Niederberger, H. L.; Kneip, M.; Weil, F. Stud. Surf. Sci. Catal. 1991, 61, 313. |
| [5] | Botes, F. G.; B?hringer, W. Appl. Catal. A 2004, 267, 217. |
| [6] | Guczi, L.; Kiricsi, I. Appl. Catal. A 1999, 186, 375. |
| [7] | Wei, J.; Ge, Q. J.; Yao, R. W.; Wen, Z. Y.; Fang, C. Y.; Gu, L. S.; Xu, H. Y.; Sun, J. Nat. Commun. 2017, 8, 15174. |
| [8] | Lin, Q. H.; Zhang, Q. D.; Yang, G. H.; Chen, Q. J.; Li, J.; Wei, Q. H.; Tan, Y. S.; Wan, H. L.; Tsubaki, N. J. Catal. 2016, 344, 378. |
| [9] | Jin, Y. Z.; Yang, G. H.; Chen, Q. J.; Niu, W. Q.; Lu, P.; Yoneyama, Y.; Tsubaki, N. J. Membr. Sci. 2015, 475, 22. |
| [10] | Pour, A. N.; Zamani, Y.; Tavasoli, A.; Shahri, S. M. K.; Taheri, S. A. Fuel 2008, 87, 2004. |
| [11] | Wang, S.; Yin, Q.; Guo, J.; Ru, B.; Zhu, L. Fuel 2013, 108, 597. |
| [12] | Maddi, B.; Davidson, S.; Job, H.; Dagle, R.; Guo, M.; Gray, M.; Ramasamy, K. K. Catal. Lett. 2020, 151, 526. |
| [13] | Bao, J.; He, J. J.; Zhang, Y.; Yoneyama, Y.; Tsubaki, N. Angew. Chem. Int. Ed. 2008, 47, 353. |
| [14] | Xing, C.; Shen, W. Z.; Yang, G. H.; Yang, R. Q.; Lu, P.; Sun, J.; Yoneyama, Y.; Tsubaki, N. Catal. Commun. 2014, 55, 53. |
| [15] | Schulz, H.; van Steen, E.; Claeys, M. Stud. Surf. Sci. Catal. 1994, 81, 455. |
| [16] | Lü, J. Z.; Hu, R. Z.; Zhuo, O.; Xu, B. L.; Yang, L. J.; Wu, Q.; Wang, X. Z.; Fan, Y. N.; Hu, Z. Acta Chim. Sinica 2014, 72, 1017. (in Chinese) |
| [16] | (吕金钊, 胡仁之, 卓欧, 许波连, 杨立军, 吴强, 王喜章, 范以宁, 胡征, 化学学报, 2014, 72, 1017.) |
| [17] | Yang, X. P.; Guo, X. X.; Zhang, C. H.; Wang, X. P.; Yang, Y.; Li, Y. W. Acta Chim. Sinica 2017, 75, 360. (in Chinese) |
| [17] | (杨向平, 郭晓雪, 张成华, 王小萍, 杨勇, 李永旺, 化学学报, 2017, 75, 360.) |
| [18] | Cho, K. M.; Park, S. Y.; Seo, J. G.; Youn, M. H.; Baeck, S. H.; Jun, K. W.; Chung, J. S.; Song, I. K. Appl. Catal. B 2008, 83, 195. |
| [19] | Sun, B.; Yu, G. B.; Lin, J.; Xu, K.; Pei, Y.; Yan, S. R.; Qiao, M. H.; Fan, K. N.; Zhang, X. X.; Zong, B. N. Catal. Sci. Technol. 2012, 2, 1625. |
| [20] | Smith, A. J.; Trimm, D. L. Annu. Rev. Mater. Res. 2005, 35, 127. |
| [21] | Xu, K.; Cheng, Y.; Sun, B.; Pei, Y.; Yan, S. R.; Qiao, M. H.; Zhang, X. X.; Zong, B. N. Acta Phys.-Chim. Sin. 2015, 31, 1137. (in Chinese) |
| [21] | (许可, 程义, 孙博, 裴燕, 闫世润, 乔明华, 张晓昕, 宗保宁, 物理化学学报, 2015, 31, 1137.) |
| [22] | Fan, J. G.; Zong, B. N.; Zhang, X. X.; Meng, X. K.; Mu, X. H.; Yu, G. B.; Qiao, M. H.; Fan, K. N. Ind. Eng. Chem. Res. 2008, 47, 5918. |
| [23] | He, J. J.; Liu, Z. L.; Yoneyama, Y.; Nishiyama, N.; Tsubaki, N. Chem. Eur. J. 2006, 12, 8296. |
| [24] | Li, H. J.; Zhang, P. P.; Guo, L. S.; He, Y. L.; Zeng, Y.; Thongkam, M.; Natakaranakul, J.; Kojima, T.; Reubroycharoen, P.; Vitidsant, T.; Yang, G. H.; Tsubaki, N. ChemSusChem 2020, 13, 2060. |
| [25] | de Smit, E.; Weckhuysen, B. M. Chem. Soc. Rev. 2008, 37, 2758. |
| [26] | Yang, X. L.; Wang R. F.; Yang, J.; Qian, W. X.; Zhang, Y. R.; Li, X. N.; Huang, Y. Q.; Zhang, T.; Chen, D. ACS. Catal. 2020, 10, 3797. |
| [27] | Grenoble, D. C.; Estadt, M. M.; Ollis, D. F. J. Catal. 1981, 67, 90. |
/
| 〈 |
|
〉 |