

Theoretical Study on the Structural Stability and Oxygen Ion Oxidation of Al-doped Lithium-ion Battery Layered Cathode Li(Li0.17Ni0.17Al0.04Fe0.13Mn0.49)O2
Received date: 2021-04-26
Online published: 2021-07-27
Supported by
Inner Mongolia Natural Science Foundation(2021MS02003)
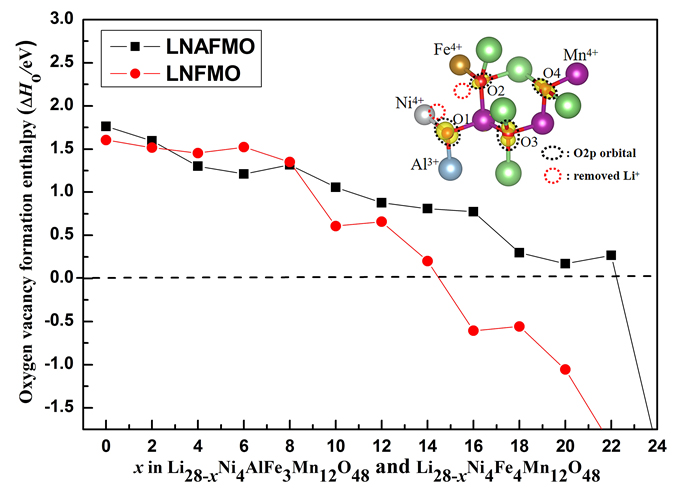
In lithium-ion battery, lithium rich layered transition metal oxides materials are the next generation of lithium ion cathode materials with high practical specific capacity. The specific capacity and structural stability of cathode materials are two important factors. In order to improve the overall performance of batteries, some strategies such as chemical modifications, surface coating and material composite are used. Among them element doping is an effectively method to improve the electrochemical stability of lithium-rich cathode materials. Li(Li0.17Ni0.17Al0.04Fe0.13Mn0.49)O2 (LNAFMO) as a layered cobalt-free cathode material was selected as the research object. Geometrical structures including the lattice parameters, M―O bond length (where M stands for transition metal) and O―O bond length, electronic structures including oxidation processes of transition metal and oxygen ion, oxygen release enthalpies, delithium formation energies, delithium voltage and the effect of doping element Al are investigated by a GGA (generalized gradient approximation)+U (Hubbard U value) method. Calculated results show that Ni2+ ions in the LNAFMO system are first oxidized, then Fe3+ and finally O2- during the charging. Oxygen ions with linear Al-O-Li configurations in the LNAFMO system participate in charge compensation besides those with linear Li-O-Li and Fe-O-Li configurations, different from the Li(Li0.17Ni0.17Fe0.17Mn0.49)O2 (LNFMO) system without Al doping. The doping of Al can suppress the release of oxygen, improving the structural stability of the system and the cycling performance of the battery. The doping of Al increases the special capacity of LNFMO system (246 mAh•g-1), in which the contribution of Ni/Fe transition metal and oxygen ions to the capacity is equal. The internal mechanism between the oxidation of transition metals and oxygen ions and the geometrical structures in the system has been revealed from a microscopic perspective. This work would provide a theoretical basis for the design of a low cost, high energy density and high cycle performance lithium-ion battery cathode material.
Kai Qiu , Mingxia Yan , Shouwang Zhao , Shengli An , Wei Wang , Guixiao Jia . Theoretical Study on the Structural Stability and Oxygen Ion Oxidation of Al-doped Lithium-ion Battery Layered Cathode Li(Li0.17Ni0.17Al0.04Fe0.13Mn0.49)O2[J]. Acta Chimica Sinica, 2021 , 79(9) : 1146 -1153 . DOI: 10.6023/A21040178
| [1] | Huang, X. J. Materials China 2010, 29, 46. (in Chinese) |
| [1] | ( 黄学杰, 中国材料进展, 2010, 29, 46.) |
| [2] | Xu, H.; Zhong, H. Chinese J. Inorg. Chem. 2004, 19, 497. (in Chinese) |
| [2] | ( 许惠, 钟辉, 无机化学学报, 2004, 19, 497.) |
| [3] | Yin, D.; Daobin, M.; Borong, W.; Rui, W. Z. Appl. Energ. 2017, 195, 586. |
| [4] | Ke, W.; Wei, Y. M.; Zhang, X. Appl. Energ. 2013, 104, 105. |
| [5] | Yang, Z. G.; Zhang, J. L.; Kintner-Meyer, M. C. W.; Lu, X. C.; Choi, D. W.; Lemmon, J. P.; Liu, J. Chem. Rev. 2011, 111, 3577. |
| [6] | Feng, C. S.; Rui, X.; Hong, W. H. Appl. Energ. 2016, 162, 1399. |
| [7] | Yan, J. D. Chinese J. Aeronayt. 2014, 35, 2767. (in Chinese) |
| [7] | ( 闫金定, 航空学报, 2014, 35, 2767.) |
| [8] | Wang, L.; Gao, P. Z.; Li, D. Y.; Huang, S. T.; Xiao, H. N. Bull. Chin. Ceram. Soc. 2013, 32, 77. (in Chinese) |
| [8] | ( 王玲, 高朋召, 李冬云, 黄诗婷, 肖汉宁, 硅酸盐通报, 2013, 32, 77.) |
| [9] | Deng, B.; Sun, W.; Wang, H.; Chen, T.; Li, X.; Qu, M.; Peng, G. Acta Chim. Sinica 2018, 76, 259. (in Chinese) |
| [9] | ( 邓邦为, 孙万琦, 王昊, 陈滔, 李璇, 瞿美臻, 彭工厂, 化学学报, 2018, 76, 259.) |
| [10] | Ma, C.; Lv, Y. C.; Li, H. Energy Storage Science and Technology 2014, 3, 53. (in Chinese) |
| [10] | ( 马璨, 吕迎春, 李泓, 储能科学与技术, 2014, 3, 53.) |
| [11] | Li, H.; Zhang, L. P.; Yu, X. J. Bull. Chin. Ceram. Soc. 2012, 31, 1486. (in Chinese) |
| [11] | ( 李恒, 张丽鹏, 于先进, 硅酸盐通报, 2012, 31, 1486.) |
| [12] | Li, W.; Tian, W. H.; Qi, L. Inorg. Chem. Ind. 2015, 47, 1. (in Chinese) |
| [12] | ( 李卫, 田文怀, 其鲁, 无机盐工业, 2015, 47, 1.) |
| [13] | Nitta, N.; Wu, F. X.; Lee, J. T.; Yushin, G. Mater. Today 2015, 18, 252. |
| [14] | Luo, K.; Roberts, M. R.; Hao, R.; Guerrini, N.; Pickup, D. M.; Liu, Y. S.; Edstrom, K.; Guo, J. H.; Chadwick, A. V.; Duda, L. C.; Bruce, P. G. Nat. Chem. 2016, 8, 684. |
| [15] | Li, B.; Xia, D. G. Adv. Mater. 2017, 29, 1. |
| [16] | Cheng, X. L.; Wei, H. Z.; Hao, W. J.; Li, H. Y.; Si, H. N.; An, S. L.; Zhu, W. T.; Jia, G. X.; Qiu, X. P. ChemSusChem 2019, 12, 1162. |
| [17] | Zhuo, Z. Q.; Dai, K. H.; Qiao, R. M.; Wang, R.; Wu, J. P.; Liu, Y. L.; Peng, J. Y.; Chen, L. Q.; Chuang, Y. D.; Pan, F.; Shen, Z. X.; Liu, G.; Li, H.; Devereaux, T. P.; Yang, W. L. Joule 2021, 5, 975. |
| [18] | Wang, Z. X.; Chen, L. Q.; Huang, X. J. Prog. Chem. 2011, 23, 284. (in Chinese) |
| [18] | ( 王兆翔, 陈立泉, 黄学杰, 化学进展, 2011, 23, 284.) |
| [19] | Hassoun, J.; Kim, J.; Lee, D. J.; Jung, H. G.; Lee, S. M.; Sun, Y. K.; Scrosati, B. J. Power Sources 2012, 202, 308. |
| [20] | Ma, Q. X.; Meng, J. X.; Yang, L.; Cao, W. Chin. J. Nonferrous Met. 2013, 23, 456. (in Chinese) |
| [20] | ( 马全新, 孟军霞, 杨磊, 曹文, 中国有色金属学报, 2013, 23, 456.) |
| [21] | Li, Z.; Wang, Z.; Ban, L. Q.; Wang, J. T.; Lu, S. G. Acta Chim. Sinica 2019, 77, 1115. (in Chinese) |
| [21] | ( 李钊, 王忠, 班丽卿, 王建涛, 卢世刚, 化学学报, 2019, 77, 1115.) |
| [22] | Gao, Y. R.; Wang, X. F.; Ma, J.; Wang, Z. X.; Chen, L. Q. Chem. Mater. 2015, 27, 3456. |
| [23] | Nakahara, K.; Tabuchi, M.; Kuroshima, S.; Toda, A.; Tanimoto, K.; Nakano, K. J. Electrochem. Soc. 2012, 159, A1398. |
| [24] | Tabuchi, M.; Nabeshima, Y.; Takeuchi, T.; Tatsumi, K.; Imaizumi, J.; Nitta, Y. J. Power Sources 2010, 195, 834. |
| [25] | Laisa, C. P.; Kumar, A. K. N.; Chandrasekaran, S. S.; Murugan, P.; Lakshminarasimhan, N.; Govindaraj, R.; Ramesha, K. J. Power Sources 2016, 324, 462. |
| [26] | Gao, Y. R; Wang, X. F.; Ma, J.; Wang, Z. X.; Chen, L. Q. Chem. Mater. 2015, 27, 3456. |
| [27] | Zheng, J.; Gu, M.; Xiao, J.; Polzin, B. J.; Yan, P.; Chen, X.; Wang, C.; Zhang, G. Chem. Mater. 2014, 26, 6320. |
| [28] | Kang, S. H.; Johnson, C. S.; Vaughey, J. T.; Amine, K.; Thackeray, M. M. J. Electrochem. Soc. 2006, 153, A1186. |
| [29] | Qing, R. P.; Shi, J. L.; Xiao, D. D.; Zhang, X. D.; Yin, Y. X.; Zhai, Y. B.; Gu, L.; Guo, Y. G. Adv. Energy Mater. 2016, 6, 1501914. |
| [30] | Jang, Y. I.; Chiang, Y. M. Solid State Ionics 2000, 130, 53. |
| [31] | Wang, L. Z.; Wang, H. F.; Gu, S. H.; Wang, S. X. Battery Bimonthly 2005, 2, 155. (in Chinese) |
| [31] | ( 王力臻, 王红芳, 谷书华, 王树新, 电池, 2005, 2, 155.) |
| [32] | He, A. Z. Inorg. Chem. Ind. 2017, 49, 74 (in Chinese) |
| [32] | ( 何爱珍, 无机盐工业, 2017, 49, 74.) |
| [33] | Ren, X. Q.; Li, D. L.; Zhao, Z. Z.; Chen, G. Q.; Zhao, K.; Kong, X. Z.; Li, T. X. Acta Chim. Sinica 2020, 78, 1268. (in Chinese) |
| [33] | ( 任旭强, 李东林, 赵珍珍, 陈光琦, 赵坤, 孔祥泽, 李童心, 化学学报, 2020, 78, 1268.) |
| [34] | Liang, Y. L. M.S. Thesis, Harbin Institute of Technology, Harbin, 2010. (in Chinese) |
| [34] | ( 梁一林, 硕士论文, 哈尔滨工业大学, 哈尔滨, 2010.) |
| [35] | Wei, H. Z.; Cheng, X. L.; Fan, H. W.; Shan, Q.; An, S. L.; Qiu, X. P.; Jia, G. X. ChemSusChem 2019, 12, 2471. |
| [36] | Wei, H. Z. M.S. Thesis, Inner Mongolia University of Science and Technology, Baotou, 2019. (in Chinese) |
| [36] | ( 卫河转, 硕士论文, 内蒙古科技大学, 包头, 2019.) |
| [37] | Cao, T.; Shi, C.; Zhao, N.; He, C. N.; Li, J. J.; Liu, E. Z. J. Phys. Chem. C 2015, 119, 28749. |
| [38] | Luo, K.; Roberts, M. R.; Guerrini, N.; Tapia-Ruiz, N.; Hao, R.; Massel, F.; Pickup, D. M,; Ramos, S.; Liu, Y. S.; Guo, J. H.; Chadwick, A. V.; Duda, L. C.; Bruce, P. G. J. Am. Chem. Soc. 2016, 138, 11211. |
| [39] | Seo, D. H.; Lee, J.; Urban, A.; Malik, R.; Kang, S.; Ceder, G. Nat. Chem. 2016, 8, 692. |
| [40] | Kresse, G.; Furthmüller J. Comp. Mater. Sci. 1996, 6, 15. |
| [41] | Song, L. B.; Li, A. X.; Xiao, Z. L.; Chi, Z. Z.; Cao, Z. J. Chem. Ind. Eng. 2019, 70, 2051. (in Chinese) |
| [41] | ( 宋刘斌, 黎安娴, 肖忠良, 池振振, 曹忠, 化工学报, 2019, 70, 2051.) |
| [42] | Zhang, Y. J. M.S. Thesis, Shandong University, Jinan, 2013. (in Chinese) |
| [42] | ( 张雍家, 硕士论文, 山东大学, 济南, 2013.) |
| [43] | Xu, B.; Wang, Z. Met. Funct. Mater. 2012, 19, 40. (in Chinese) |
| [43] | ( 徐本刚, 王忠, 金属功能材料, 2012, 19, 40.) |
| [44] | Dudarev, S. L.; Botton, G. A.; Savrasov, S. Y. Phys. Rev. B 1998, 57, 1505. |
| [45] | Xu, Y. H.; Yi, G. P.; Zuo, J. P. Prog. Chem. 2008, 11, 1827. (in Chinese) |
| [45] | ( 徐宇虹, 尹鸽平, 左朋建, 化学进展, 2008, 11, 1827.) |
| [46] | De Dompablo, M. E. A. Y.; Ceder, G.; J. Power. Sources 2003, 119-121, 654. |
/
| 〈 |
|
〉 |