

Construction of Three-dimensional Ion-conducting Channels of Poly(vinylidene fluoride) Membranes and Their Performance in Vanadium Redox Flow Battery
Received date: 2021-05-24
Online published: 2021-08-10
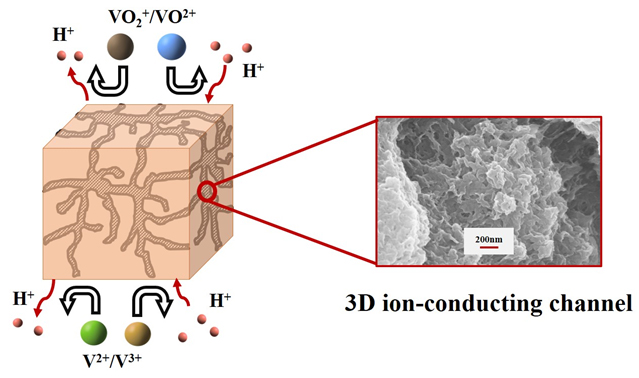
With the increasing demand of renewable energy, large-scale energy storage technology has attracted extensive attention. Vanadium redox flow battery (VRFB), benefiting from its adjustable capacity, high safety and long life, has become one of the fastest developing batteries for large-scale energy storage. Ion exchange membrane is a key component of VRFB, which notably affects the efficiency, cost and stability of the batteries. However, as the most commonly used membrane in VRFBs, Nafion shows shortages of high vanadium permeability and high economic cost, which largely hinder the commercial application of VRFBs. In order to develop low-cost, durable and high-performance ion exchange membranes for flow batteries, in this work, dual-porous poly(vinylidene fluoride) ion exchange membranes have been developed, in which polyethylene glycol (PEG) and polyvinylidene pyrrolidone (PVP) are applied as the template and stabilizer molecules, respectively. As a result, three-dimensional ion-conducting network has been successfully built in the poly(vinylidene fluoride) (PVDF) matrix which enable fast proton conduction and ion selection. The ion-conducting channels and thereby the proton conductivity and H/V ion selectivity of the membrane can be finely controlled by simply adjusting the PEG content in the casting solution. The unique dual porous structure of the membrane is clearly observed by using a scanning electron microscope. Battery efficiency including coulombic efficiency, voltage efficiency and energy efficiency have been characterized in VRFB single cells and the results show that the prepared PVDF ion-exchange membrane possesses high coulombic efficiency exceeding 98% and energy efficiency as high as 83.5% at a current density of 100 mA•cm-2, which are comparable to that of Nafion membranes. Moreover, the prepared PVDF ion-exchange membranes illustrate excellent chemical stability after the chemical stability test lasting for 30 d. In a word, due to the very low cost of the polymer material, excellent chemical stability and good performance of the dual-porous PVDF membranes, the novel ion exchange membrane shows great prospect for the application in VRFBs.
Feiran Wang , Fengjing Jiang . Construction of Three-dimensional Ion-conducting Channels of Poly(vinylidene fluoride) Membranes and Their Performance in Vanadium Redox Flow Battery[J]. Acta Chimica Sinica, 2021 , 79(9) : 1123 -1128 . DOI: 10.6023/A21050231
| [1] | Turner, J. A. Science 1999, 285, 687. |
| [2] | Qing, G. L.-T.; Guo, W.-N.; Liu, P.; Wang, B.-G. J. Electrochem. 2015, 21, 449. (in Chinese) |
| [2] | ( 青格勒图, 郭伟男, 刘平, 王保国, 电化学, 2015, 21, 449.) |
| [3] | Kim, S.; Yan, J.; Schwenzer, B.; Zhang, J.; Li, L.; Liu, J.; Yang, Z.; Hickner, M. A. Electrochem. Commun. 2010, 12, 1650. |
| [4] | Zhao, Y.-T.; Xi, J.-Y.; Teng, X.-G.; Wu, Z.-H.; Qiu, X.-P. Acta Chim. Sinica 2011, 69, 132. (in Chinese) |
| [4] | ( 赵永涛, 席靖宇, 腾祥国, 武增华, 邱新平, 化学学报, 2011, 69, 132.) |
| [5] | Li, L.; Kim, S.; Wang, W.; Vijayakumar, M.; Nie, Z.; Chen, B.; Zhang, J.; Xia, G.; Hu, J.; Graff, G. Adv. Energy Mater. 2011, 1, 394. |
| [6] | Ulaganathan, M.; Aravindan, V.; Yan, Q.; Madhavi, S.; Skyllas-Kazacos, M.; Lim, T. M. Adv. Energy Mater. 2016, 3, 1500309. |
| [7] | Wei, X.; Nie, Z.; Luo, Q.; Li, B.; Chen, B.; Simmons, K.; Sprenkle, V.; Wei, W. Adv. Energy Mater. 2014, 3, 1215. |
| [8] | Wan, Y. H.; Sun, J.; Jiang, H. R.; Fan, X. Z.; Zhao, T. S. J. Power Sources 2021, 489, 229502. |
| [9] | Wang, F.-R.; Jiang, F.-J. Prog. Chem. 2021, 33, 462. (in Chinese) |
| [9] | ( 王斐然, 蒋峰景, 化学进展, 2021, 33, 462.) |
| [10] | Zhou, X.; Xue, R.; Zhong, Y.; Zhang, Y.; Jiang, F. J. Membrane Sci. 2020, 595, 117614. |
| [11] | Zhang, B.-G.; Zhang, S.-H.; Xing, D.-B.; Yin, C.-X.; Han, R.-L.; Jian, X.-G. Acta Chim. Sinica 2011, 69, 2583. (in Chinese) |
| [11] | ( 张本贵, 张守海, 邢东博, 尹春香, 韩润林, 蹇锡高, 化学学报, 2011, 69, 2583.) |
| [12] | Li, L.-Q.; Chen, J.-W.; Lu, H.; Jiang, C.-P.; Gao, S.; Yang, X.; Lian, X.-J.; Liu, X.-J.; Wang, R.-L. Acta Chim. Sinica 2009, 67, 2785. (in Chinese) |
| [12] | ( 李良琼, 陈金伟, 鲁惠, 姜春萍, 高山, 杨鑫, 练晓娟, 刘效疆, 王瑞林, 化学学报, 2009, 67, 2785.) |
| [13] | Ling, L. A.; Min, X. A.; Dh, B.; Shan, R. A.; Sw, A.; Ym, A. J. Membrane Sci. 2019, 585, 230. |
| [14] | Fu, L.; Hashim, N. A.; Liu, Y.; Abed, M.; Li, K. Fuel Energ. Abstr. 2011, 375, 1. |
| [15] | Li, B.-Y.; Liu, Z.-H.; Wang, B.-G. CIESC J. 2017, 68, 732. (in Chinese) |
| [15] | ( 李冰洋, 刘珍豪, 王保国, 化工学报, 2017, 68, 732.) |
| [16] | Yang, X.; Zhu, H.; Jiang, F.; Zhou, X. J. Power Sources 2020, 473, 228586. |
| [17] | Lai, Y.; Wan, L.; Wang, B. Membranes 2019, 9, 89. |
| [18] | Mai, Z.; Zhang, H.; Li, X.; Xiao, S.; Zhang, H. J. Power Sources 2011, 196, 5737. |
| [19] | Ling, L.; Xiao, M.; Han, D.; Ren, S.; Meng, Y. J. Membrane Sci. 2019, 585, 230. |
| [20] | Luo, X.; Lu, Z.; Xi, J.; Wu, Z.; Zhu, W.; Chen, L.; Qiu, X. J. Phys. Chem. B 2005, 109, 20310. |
| [21] | Li, B.; Wang, B.; Liu, Z.; Qing, G. J. Membrane Sci. 2016, 111. |
| [22] | Xue, R.; Jiang, F.; Wang, F.; Zhou, X. J. Power Sources 2019, 449, 227475. |
| [23] | Sun, C.; Jian, C.; Zhang, H.; Xi, H.; Luo, Q. J. Power Sources 2010, 195, 890. |
| [24] | Jiang, B.; Wu, L.; Yu, L.; Qiu, X.; Xi, J. J. Membrane Sci. 2016, 510, 18. |
| [25] | Zhang, Y.; Zhou, X.; Xue, R.; Yu, Q.; Jiang, F.; Zhong, Y. Int. J. Hydrogen Energ. 2019, 44, 5997. |
| [26] | Zhao, Y.; Zhang, H.; Xiao, C.; Lin, Q.; Li, X. Nano Energy 2018, 48, 353. |
/
| 〈 |
|
〉 |