

Synthesis of Ag2S Based Quantum Dots with Near-infrared-II Fluorescence Emission in Water
Received date: 2021-07-16
Online published: 2021-08-13
Supported by
National Natural Science Foundation of China(21975191); National Natural Science Foundation of China(21805218); National Natural Science Foundation of China(51873168); National innovation and Entrepreneurship Training Program for College Students(202110497008)
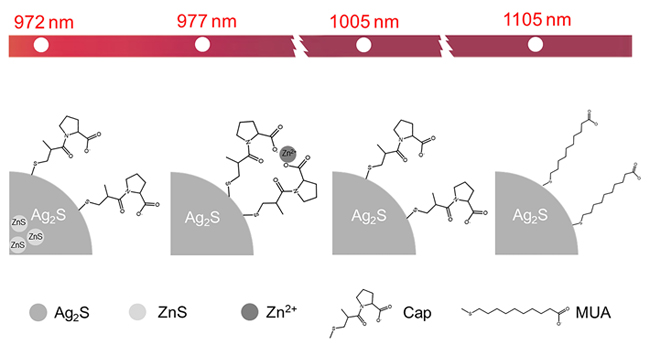
Growing numbers of quantum dots (QDs) have been applied in biological fluorescence imaging, especially those QDs with near-infrared-II fluorescence emission, avoiding the interference of autofluorescence of biological tissues, have broad application prospects in the field of fluorescence imaging. In particularly, Ag2S quantum dots (Ag2S QDs) have drew great attention of researchers in biological fluorescence imaging due to the near-infrared fluorescence emission, large Stokes shift, good light and chemical stability. However, the Ag2S QDs synthesized in organic phase usually experienced poor water solubility and biocompatibility, while the fluorescence of the Ag2S QDs synthesized in aqueous phase is difficult to reach the near-infrared-II region, which severely restricts the promotion of Ag2S QDs in biological fluorescence imaging. Therefore, it is of great significance to optimize and explore the water-phase synthesis method of Ag2S based quantum dots with near-infrared-II fluorescence emission. The goal of this work is to synthesize Ag2S QDs with near-infrared-II region fluorescence emission by aqueous phase method. First, captopril modified Ag2S QDs (Ag2S@Cap QDs) were prepared in water, and its fluorescence emission peak lied in the near-infrared-II region at 1005 nm. Then a series of Zn:Ag2S@Cap QDs were prepared in aqueous by introducing ZnS to the Ag2S core. The results showed that the fluorescence emission peaks of Zn:Ag2S@Cap QDs blue shifted in a core-doping ZnS-dose dependent manner. Next, a series of Ag2S@Cap-Zn QDs were prepared in aqueous by introducing Zn2+ to the ligand-shell of Ag2S QDs. The fluorescence emission peaks of these Ag2S@Cap-Zn QDs also blue shifted in a Zn2+-dose dependent manner. Finally, Ag2S@MUA QDs were prepared by replacing the shell ligand from Cap to 11-mercaptoundecanoic acid (MUA). The fluorescence emission peak of these Ag2S@MUA QDs red shifted to 1105 nm, locating in the near-infrared-II region, and the width of the fluorescence curve at half-maximum height became narrower, implying better application prospect in biological fluorescence imaging. This work not only successfully prepared a kind of Ag2S QDs with near-infrared-II fluorescence emission in aqueous phase, but also opened an avenue for the preparation and optimization of semiconductor QDs with near-infrared fluorescence.
Meng Yu , Zijun Zhang , Guowei Zhu , Zhenhua Gu , Yulin Duan , Liangchong Yu , Guanbin Gao , Taolei Sun . Synthesis of Ag2S Based Quantum Dots with Near-infrared-II Fluorescence Emission in Water[J]. Acta Chimica Sinica, 2021 , 79(10) : 1281 -1285 . DOI: 10.6023/A21070333
| [1] | Sang, R.; Xu, X.; Wang, Q.; Fan, Q.; Huang, W. Acta Chim. Sinica 2020, 78, 901. (in Chinese) |
| [1] | (桑若愚, 许兴鹏, 王其, 范曲立, 黄维, 化学学报, 2020, 78, 901.) |
| [2] | Zhang, M.; Yue, J.; Ran, C.; Ma, Z.; Dai, H. Proc. Natl. Acad. Sci. Belarus-Agrar. Ser. 2018, 115, 201806153. |
| [3] | Gao, G.; Gong, D.; Zhang, M.; Sun, T. Acta Chim. Sinica 2016, 74, 363. (in Chinese) |
| [3] | (高冠斌, 龚德君, 张明曦, 孙涛垒, 化学学报, 2016, 74, 363.) |
| [4] | Alivisatos, A. P. Science 1996, 271, 933. |
| [5] | Niu, Z. Natl. Sci. Rev. 2017, 4, 167. |
| [6] | Mo, D.; Hu, L. Zeng, G.; Chen, G.; Wan, J.; Yu, Z.; Huang, Z.; He, K.; Zhang, C.; Cheng, M. Appl. Microbiol. Biotechnol. 2017, 101, 2713. |
| [7] | Zhu, J.; Tang, Y.; Wang, G.; Mao, J.; Liu, Z.; Sun, T.; Wang, M.; Chen, D.; Yang, Y.; Li, J.; Deng, Y.; Yang, S. ACS Appl. Mater. Interfaces 2017, 9, 14470. |
| [8] | Feng, Y.; Wang, X.; Huang, J.; Dong, P.; Wang, C. Chem. Eng. J. 2020, 390, 124525. |
| [9] | Murray, C. B.; Norris, D. J.; Bawendi, M. G. J. Am. Chem. Soc. 1993, 115, 8706. |
| [10] | Hocaoglu, I.; Natali, M.; Erdem, R.; Ozen, C.; Kurt, A.; Sennaroglu, A.; Acar, H. J. Mater. Chem. 2012, 22, 14674. |
| [11] | Du, Y.; Xu, B.; Fu, T.; Cai, M.; Li, F.; Zhang, Y.; Wang, Q. J. Am. Chem. Soc. 2010, 132, 1470. |
| [12] | Luo, X.; Chen, M.; Yang, Q. Acta Chim. Sinica 2020, 78, 373. (in Chinese) |
| [12] | (罗兴蕊, 陈敏文, 杨晴来, 化学学报, 2020, 78, 373.) |
| [13] | Wang, J.; Yang, F.; Gao, G.; Sun, T. J. Inorg. Mater. 2019, 34, 1156. |
| [13] | (王君诚, 杨菲菲, 高冠斌, 孙涛垒, 无机材料学报, 2019, 34, 1156.) |
| [14] | Gan, Z.; Liu, Y.; Wang, L.; Jiang, S.; Xia, N.; Yan, Z.; Wu, X.; Zhang, J.; Gu, W.; He, L.; Dong, J.; Ma, X.; Kim, J.; Wu, Z.; Xu, Y.; Li, Y.; Wu, Z. Nat. Commun. 2020, 11, 5572. |
| [15] | Guo, J.; Guo, M.; Wang, F.; Jin, W.; Chen, C.; Liu, H.; Li, Y. Angew. Chem., Int. Ed. 2020, 59, 16712. |
| [16] | Thiessen, A. N.; Zhang, L.; Oliynyk, A. O.; Yu, H.; O'Connor, K. M.; Meldrum, A.; Veinot, J. G. C. Chem. Mater. 2020, 32, 6838. |
| [17] | Medintz, I. L.; Uyeda, H. T.; Goldman, E. R.; Mattoussi, H. Nat. Mater. 2005, 4, 435. |
| [18] | Valizadeh, A.; Mikaeili, H.; Samiei, M.; Farkhani, S. M.; Zarghami, N.; Kouhi, M.; Akbarzadeh, A.; Davaran, S. Nanoscale Res. Lett. 2012, 7, 480. |
| [19] | Peng, Z. A.; Peng, X. J. Am. Chem. Soc. 2001, 123, 183. |
| [20] | Anc, M. J.; Pickett, N. L.; Gresty, N. C.; Harris, J. A.; Mishra, K. C. ECS J. Solid State Sci. Technol. 2013, 2, 3071. |
| [21] | Xu, Y.; Zhao, Y.; Zhang, Y.; Cui, Z.; Wang, L.; Fan, C.; Gao, J.; Sun, Y. Acta Chim. Sinica 2018, 76, 393. (in Chinese) |
| [21] | (徐毅, 赵彦, 张叶俊, 崔之芬, 王丽华, 樊春海, 高基民, 孙艳红, 化学学报, 2018, 76, 393.) |
/
| 〈 |
|
〉 |