

Preparation of Efficient Pd/MgAl-LDO@Al2O3 Catalyst for Phenol Hydrogenation to Cyclohexanone
Received date: 2021-07-24
Online published: 2021-10-09
Supported by
National Key Research and Development Plan(2017YFE0301502); Special Fund for Basic Scientific Research Operations of Central Universities(JD2018)
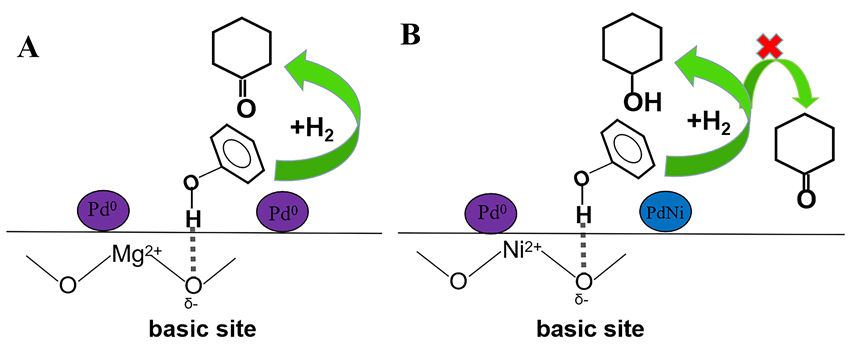
Cyclohexanone is an important intermediate for the synthesis of nylon, but the direct hydrogenation reaction of phenol to cyclohexanone can easily generate cyclohexanol. In this paper, the in situ synthesis strategy is used to grow the layered double hydrotalcites on alumina. Compared with the pristine alumina, the modified support has increased specific surface area, average pore size and abundant acid-base sites on the surface. Pd/MgAl-LDO@Al2O3 catalyst was then prepared by the calcination of precursor with layered structure, followed by the impregnation of Pd. The catalyst is used for the selective hydrogenation of phenol (reaction conditions: phenol/Pd=1000 mol/mol, 80 ℃, 0.4 MPa H2). The obtained Pd/MgAl-LDO@Al2O3 shows enhanced catalytic performance under a high substrate/Pd ratio. Detailly, when the phenol conversion is 97%, the cyclohexanone selectivity can reach 88%. High-resolution transmission electron microscopy (HRTEM) and X-ray photoelectron spectroscopy (XPS) analysis are performed to characterize the catalyst, and it is found that in situ growth of hydrotalcite-like structure on alumina can effectively decrease the average particle size of Pd and then increase the dispersion of active component. Moreover, when Ni is introduced into the layered structure, PdNi alloy structure could be obtained by the easy reduction process of using NaBH4. Kinetics study indicates the energy barrier of the phenol hydrogenation reaction over Pd/NiAl-LDO@Al2O3 is lower than that over Pd/MgAl-LDO@Al2O3 due to the formation of PdNi alloy. But the alloy structure leads to an obvious decrease of cyclohexanone selectivity. According to the product distribution and characterization results, a possible mechanism is proposed. Phenol is adsorbed on the surface of the basic sites on the Pd/MgAl-LDO@Al2O3 catalyst in a non-coplanar manner, which is conducive to the selective hydrogenation of phenol to produce cyclohexanone and inhibits the over-hydrogenation of cyclohexanone. After repeated use for 5 times, the Pd/MgAl-LDO@Al2O3 catalyst shows excellent stability. The phenol conversion could keep at ca. 90% and the cyclohexanone selectivity maintains ca. 90%.
Min Zhao , Xue Wang , Yanan Liu , Yufei He , Dianqing Li . Preparation of Efficient Pd/MgAl-LDO@Al2O3 Catalyst for Phenol Hydrogenation to Cyclohexanone[J]. Acta Chimica Sinica, 2021 , 79(12) : 1518 -1525 . DOI: 10.6023/A21070343
| [1] | Li, B. L.; Liu, R. Y.; Liang, R. X.; Jia, Y. X. Acta Chim. Sinica 2017, 75, 448 (in Chinese). |
| [1] | ( 李保乐, 刘人荣, 梁仁校, 贾义霞, 化学学报, 2017, 75, 448.) |
| [2] | Weng, X.; Dong, J.; She, T. T.; Bai, G. Y. Journal of Hebei University 2018, 3, 239 (in Chinese). |
| [2] | ( 温昕, 董洁, 舍添添, 白国义, 河北大学学报, 2018, 3, 239.) |
| [3] | Shore, S. G.; Ding, E.; Park, C.; Keane, M. A. Catal. Commun. 2002, 3, 77. |
| [4] | Sikhwivhilu, L. M.; Coville, N. J.; Naresh, D.; Chary, K. Appl. Catal. A, 2007, 324, 52. |
| [5] | Neri, G.; Visco, A. M.; Donato, A.; Milone, C.; Malentacchi, M.; Gubitosa, G. Appl. Catal. A, 1994, 110, 49. |
| [6] | Chary, K. V. R.; Naresh, D.; Vishwanathan, V.; Sadakane, M.; Ueda, W. Catal. Commun. 2007, 8, 471. |
| [7] | Scirè, S.; Minicò, S.; Crisafulli, C. Appl. Catal. A, 2002, 235, 21. |
| [8] | Gonzalez-Velasco, J. R.; Gonzalez-Marcos, M. P.; Arnaiz, S.; Gutierrez-Ortiz, J. I.; Gutierrez-Ortiz, M. A. Ind. Eng. Chem. Res. 1995, 34, 1031. |
| [9] | Wang, Y.; Yao, J.; Li, H. R.; Su, D. S.; Antonietti, M. J. Am. Chem. Soc. 2011, 42, 939. |
| [10] | Souza, P. M. D.; Rabelo-Neto, R. C.; Borges, L. E. P.; Jacobs, G.; Davis, B. H.; Sooknoi, T.; Resasco, D.; Nornoha, F. B. ACS Catal. 2015, 4, 1318. |
| [11] | Makowski, P.; Cakan, R. D.; Antonietti, M.; Goettmann, F.; Titirici, M. M. Chem. Commun. 2008, 39, 999. |
| [12] | Li, H.; Liu, J. L.; Li, H. X. Mater. Lett. 2008, 62, 2321. |
| [13] | Xiang, Y. Z.; Ma, L.; Lu, C. S.; Zhang, Q. F.; Li, X. N. Green Chem. 2008, 10, 939. |
| [14] | Liu, H. Z.; Jiang, T.; Han, B. X.; Liang, S. G.; Zhou, Y. X. Science 2009, 326, 1250. |
| [15] | Li, X. Z.; Cheng, L.; Wang, X. Y. Res. Chem. Intermed. 2018, 45, 1249. |
| [16] | Cavani, F.; Trifirò, F.; Vaccari, A. Catal. Today 1991, 11, 173. |
| [17] | Yu, J.; Yang, Y. S.; Wei, M. Acta Chim. Sinica 2019, 77, 1129 (in Chinese). |
| [17] | ( 余俊, 杨宇森, 卫敏, 化学学报, 2019, 77, 1129.) |
| [18] | Debecker, D. P.; Gaigneaux, E. M.; Busca, G. Chem. Eur. J. 2009, 15, 3920. |
| [19] | Sivasamy, A.; Cheah, K. Y.; Fornasiero, P.; Kemausuor, F.; Zinoviev, S.; Miertus, S. ChemSusChem 2010, 2, 278. |
| [20] | Sikhwivhilu, L.; Coville, N.; Naresh, D.; Chary, K. V. R.; Vishwanathan, V. Appl. Catal. A, 2007, 324, 52. |
| [21] | Veloso, C. O.; Pérez, C. N.; Souza, B. M. D.; Lima, E. C.; Dias, A. G.; Monteiro, J. L. F.; Henriques, C. A. Micropor. Mesopor. Mater. 2008, 107, 23. |
| [22] | Brindley, G. W.; Kikkawa, S. Clay. Clay Miner. 1980, 28, 87. |
| [23] | Mahata, N.; Vishwanathan, V. Catal. Today 1999, 49, 65. |
| [24] | Zhong, J. W.; Chen, J. Z.; Chen, L. M. Catal. Sci. Technol. 2014, 4, 3555. |
| [25] | Yuan, X. Q.; Li, B. T.; Li, B.; Wang, X. J. Fuel Process. Technol. 2021, 211, 106581. |
| [26] | Chowdhury, S. R.; Maiyalagan, T.; Bhattachraya, S. K.; Gayen, A. Electrochim. Acta 2020, 342, 136028. |
| [27] | Rai, R. K.; Gupta, K.; Behrens, S.; Li, J.; Xu, Q.; Singh, S. K. ChemCatChem 2015, 7, 1806. |
/
| 〈 |
|
〉 |