

Construction and Structure-Activity Relationship of Immobilized Enzyme Reactor Based on Al-MOF-Derived Al2O3 with Hierarchical Structure
Received date: 2021-08-16
Online published: 2021-10-11
Supported by
National Natural Science Foundation of China(22003037); National Natural Science Foundation of China(22173056); Key Laboratory Project of Education Department of Shaanxi Province(20JS043); Natural Science Foundation Project(19SKY003); Natural Science Foundation Project(20SKY005); Scientific Research Team Project(20SCX01)
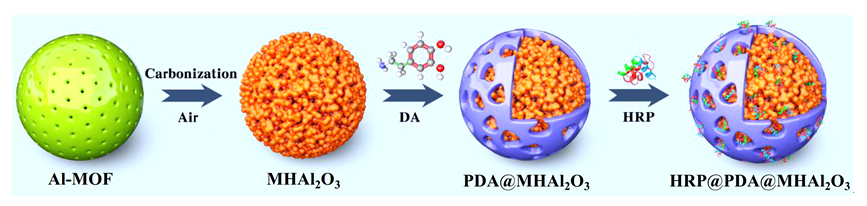
Although enzyme catalysis has many advantages such as green, high efficiency and so on, the industrial application of enzyme is often hampered by a lack of long-term operational stability and difficult to re-use. These drawbacks can generally be overcome by immobilization of the enzyme on a solid carrier. However, the immobilized enzyme reactor often can not remain all the initial catalytic activity due to the enzyme shedding during reuse, change of conformation and the difficulty for substrates to access the active site of enzyme in the carrier, etc. The contradiction between catalytic activity and stability of biological enzyme molecules supported on solid phase carriers has become a bottleneck in the design and preparation of immobilized enzyme reactors. Therefore, it is a challenging task to construct an immobilized enzyme reactor with both high catalytic activity and high stability. In this work, aluminum-based metal-organic framework (Al-MOF)-derived hierarchical porous Al2O3 (MHAl2O3) was prepared by the self-sacrificial template strategy and functionally modified with “polydopamine (PDA)” bionic membrane for entrapping horseradate peroxidase (HRP). The pore size was regulated by changing the calcination temperature of precursor. The influence of channel confinement effect of the carrier on the catalytic activity of the enzyme reactor was discussed, and the thermal stability and reusability of the HRP@PDA@MHAl2O3 was significantly improved. After 1 h at 70 ℃, 98.5% of the catalytic activity of HRP@PDA@MHAl2O3 could be maintained. After 10 times of reuse, 90.9% of the catalytic activity was still maintained. In order to analyze the structure-activity relationship of the enzyme reactor, the interaction between enzyme and substrate in the reaction catalyzed by HRP@PDA@MHAl2O3 was studied by means of enzyme kinetics and thermodynamic parameters, which showed that the affinity and specificity of the enzyme to the substrate were improved. When the enzyme reactor was applied to the catalytic degradation of aniline aerofloat in simulated wastewater, the degradation rate of 40 mg•L-1 substrate in 30 min reached 82.7% under the best reaction conditions.
Xia Gao , Huibin Pan , Zengxian He , Ke Yang , Chengfang Qiao , Yongliang Liu , Chunsheng Zhou . Construction and Structure-Activity Relationship of Immobilized Enzyme Reactor Based on Al-MOF-Derived Al2O3 with Hierarchical Structure[J]. Acta Chimica Sinica, 2021 , 79(12) : 1502 -1510 . DOI: 10.6023/A21080385
| [1] | Zhou, Y. G.; Mohamadi, R. M.; Poudineh, M.; Kermanshah, L.; Ahmed, S.; Safaei, T. S.; Stojcic, J.; Nam, R. K.; Sargent, E. H.; Kelley, S. O. Small 2016, 12, 727. |
| [2] | Chen, W.; Jin, B.; Hu, Y. L.; Lu, Y.; Xia, X. H. Small 2012, 8, 1001. |
| [3] | Cui, J. W.; He, S.; Dai, S.; Liu, L. Y.; Zhao, A.; Lu, L.; Yang, P.; Chen, J.; Huang, N. Chem. Eng. J. 2021, 424, 130392. |
| [4] | Cheng, H. P.; Hu, M. C.; Zhai, Q. G.; Li, S. N.; Jiang, Y. C. Chem. Eng. J. 2018, 347, 703. |
| [5] | Zhuang, W.; Quan, X. B.; Wang, Z. F.; Zhou, W. F.; Yang, P. P.; Ge, L.; Hernandez, B. V.; Wu, J. L.; Li, M.; Zhou, J.; Zhu, C. J.; Ying, H. J. Chem. Eng. J. 2020, 394, 125038. |
| [6] | Sankaran, R.; Show, P. L.; Chang, J. S. Biofuel. Bioprod. Bior. 2016, 10, 896. |
| [7] | Zhao, X. B.; Qi, F.; Yuan, C. L.; Du, W.; Liu, D. H. Renew. Sust. Energy Rev. 2015, 44, 182. |
| [8] | An, K. J.; Kwon, S. G.; Park, M.; Na, H. B.; Baik, S.; Yu, J. H.; Kim, D.; Son, J. S.; Kim, Y.W.; Song, I. C.; Moon, W. K.; Park, H. M.; Hyeon, T. Nano Lett. 2008, 8, 4252. |
| [9] | Xiong, S. L.; Zeng, H. C. Angew. Chem. Int. Ed. 2012, 51, 949. |
| [10] | Chen, J.; He, S. M.; Huang, B.; Zhang, L. Y.; Qiao, Z. Q.; Wang, J.; Yang, G. C.; Huang, H.; Hao, Q. L. Appl. Surf. Sci. 2018, 457, 508. |
| [11] | Journet, C.; Maser, W. K.; Bernier, P.; Loiseau, A.; Lamy de la Chapelle, M.; Lefrant, S.; Deniard, P.; Lee, R.; Fischer, J. E. Nature 1997, 388, 756. |
| [12] | Ishaq, S.; Tamime, R.; Bilad, M. R.; Khan, A. L. Sep. Purif. Technol. 2019, 210, 442. |
| [13] | Deng, Q.; Wang, R. Catal. Commun. 2019, 120, 11. |
| [14] | Zhang, W. Q.; Li, Q. Y.; Yang, X. Y.; Ma, Z.; Wang, H. H.; Wang, X. J. Acta Chim. Sinica 2017, 75, 80 (in Chinese). |
| [14] | ( 张文强, 李秋艳, 杨馨雨, 马征, 王欢欢, 王晓军, 化学学报, 2017, 75, 80.) |
| [15] | Small, L. J.; Hill, R. C.; Krumhansl, J. L.; Schindelholz, M. E.; Chen, Z.; Chapman, K. W.; Zhang, X.; Yang, S.; Schröder, M.; Nenoff, T. M. ACS Appl. Mater. Inter. 2019, 11, 27982. |
| [16] | Chang, Z.; Qiao, Y.; Yang, H. J.; Deng, H.; Zhu, X. Y.; He, P.; Zhou, H. S. Acta Chim. Sinica 2021, 79, 139 (in Chinese). |
| [16] | ( 常智, 乔羽, 杨慧军, 邓瀚, 朱星宇, 何平, 周豪慎, 化学学报, 2021, 79, 139.) |
| [17] | Wu, M. X.; Yang, Y. W. Adv. Mater. 2017, 29, 1606134. |
| [18] | Chen, X. R.; Tong, R. L.; Shi, Z. Q.; Yang, B.; Liu, H.; Ding, S. P.; Wang, X.; Lei, Q. F.; Wu, J.; Fang, W. J. ACS. Appl. Mater. Inter. 2018, 10, 2328. |
| [19] | Liu, Y.; Shao, X. X.; Kong, D. Q.; Li, G. Q.; Li, Q. S. Colloids Surf. B Biointerfaces 2021, 197, 111450. |
| [20] | Zhao, R. N.; Hu, M. C.; Li, S. N.; Zhai, Q. G.; Jiang, Y. C. Acta Chim. Sinica 2017, 75, 293 (in Chinese). |
| [20] | ( 赵睿南, 胡满成, 李淑妮, 翟全国, 蒋育澄, 化学学报, 2017, 75, 293.) |
| [21] | Nadar, S.; Rathod, V. K. Int. J. Biol. Macromol. 2018, 120, 2293. |
| [22] | Liu, X. P.; Yan, Z. Q.; Zhang, Y.; Liu, Z. W.; Sun, Y. H.; Ren, J. S.; Qu, X. G. ACS Nano 2019, 13, 5222. |
| [23] | Yao, X. F.; Li, Y. W. Chin. Sci. Bull. 2015, 60, 1906 (in Chinese). |
| [23] | ( 姚显芳, 李映伟, 科学通报, 2015, 60, 1906.) |
| [24] | Bhadra, B. N.; Vinu, A.; Serre, C.; Jhung, S. H. Mater. Today 2019, 25, 88. |
| [25] | Zhong, M.; Kong, L.; Li, N.; Liu, Y. Y.; Zhu, J.; Bu, X. H. Coord. Chem. Rev. 2019, 388, 172. |
| [26] | Salunkhe, R. R.; Kaneti, Y. V.; Yamauchi, Y. ACS Nano 2017, 11, 5293. |
| [27] | Wei, W. B.; Dong, S. Y.; Huang, G. Q.; Xie, Q.; Huang, T. L. Sens. Actuators B: Chem. 2018, 260, 189. |
| [28] | Xie, W.; Zhou, L. J.; Xu, J.; Guo, Q. L.; Jiang, F. L.; Liu, Y. Acta Phys.-Chim. Sin. 2020, 36, 1905051 (in Chinese). |
| [28] | ( 谢文, 周莲娇, 徐娟, 郭清莲, 蒋风雷, 刘义, 物理化学学报, 2020, 36, 1905051.) |
| [29] | Li, L.; Xiang, S. L.; Cao, S. Q.; Zhang, J. Y.; Ouyang, G. F.; Chen, L. P.; Su, C. Y. Nat. Commun. 2013, 4, 1774. |
| [30] | Chen, Y.; Xiao, Z.; Liu, Y.; Fan, L. Z. J. Mater. Chem. 2017, 5, 24178. |
| [31] | Gao, X.; Zhai, Q. G.; Hu, M. C.; Li, S. N.; Song, J.; Jiang, Y. C. J. Chem. Technol. Biot. 2019, 94, 1249. |
| [32] | Song, Y. C.; Hu, M. C.; Li, S. N.; Zhai, Q. G.; Jiang, Y. C. Chem. J. Chin. Univ. 2019, 40, 1805 (in Chinese). |
| [32] | ( 宋艺超, 胡满成, 李淑妮, 翟全国, 蒋育澄, 高等学校化学学报, 2019, 40, 1805.) |
| [33] | Yang, Y.; Wang, S.; Zhou, Z.; Zhang, R.; Shen, H.; Song, J.; Su, P.; Yang, Y. BioChem. Eng. J. 2018, 137, 108. |
| [34] | Cao, D. L.; Cheng, W. J.; Tao, K.; Liang, Y. X. Macromol. Res. 2018, 26, 616. |
| [35] | Xiang, L.; Xiao, T.; Mo, C. H.; Zhao, H. M.; Li, Y. W.; Li, H.; Cai, Q. Y.; Zhou, D. M.; Wong, M. H. Ecotoxicol. Environ. Saf. 2018, 154, 84. |
| [36] | Fu, P. F.; Ma, Y. H.; Lei, B. L.; Li, G.; Lin, X. F. Environ. Technol. 2021, 42, 659. |
| [37] | Gao, X.; Zhai, Q. G.; Hu, M. C.; Li, S. N.; Jiang, Y. C. Catal. Sci. Technol. 2021, 11, 2446. |
/
| 〈 |
|
〉 |