

Design, Synthesis and Properties of Azulene-Based BN-[4]Helicenes※
Received date: 2021-11-09
Online published: 2021-12-08
Supported by
National Natural Science Foundation of China(21790362); National Natural Science Foundation of China(22075310); Science and Technology Commission of Shanghai Municipality(19XD1424700); Science and Technology Commission of Shanghai Municipality(18JC1410600)
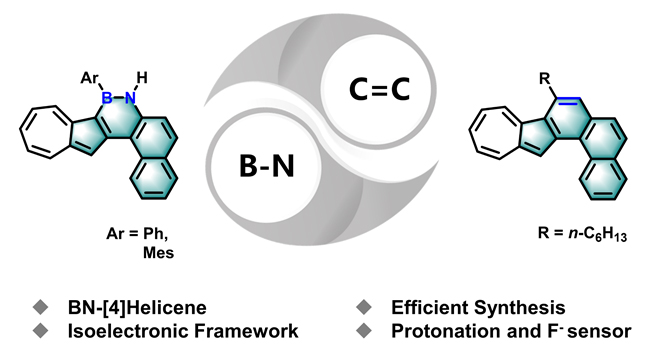
Azulene is a nonalternant and nonbenzenoid hydrocarbon with bright blue color and a dipole moment of 1.08 D, and has received increasing attention due to its unique electronic structure and physicochemical properties. Herein, we report the design and synthesis of two types of azulene-based [4]helicene 1a/1b and 2 that contain isoelectronic B—N and C=C units at the electron-rich 1-position of azulene unit, respectively. Formation of the helical scaffolds is executed by the introduction of boron and alkyne to flexible biaryl precursors, where the Lewis acidic boron and alkyne were employed as “glue” to join two subunits into fully fused scaffolds via electrophilic boronation and platinum-catalyzed cycloisomerization of alkyne at the 1-position of azulene unit, respectively. All of azulene-based helicenes were investigated by ultraviolet visible (UV-vis) absorption spectra, cyclic voltammetry (CV) measurements and density functional theory (DFT) calculations. Additionally, 1a was further characterized by single crystal structure analysis. The results suggest that the introduction of B—N unit changed the electronic structure of the conjugated aromatic framework, leading to a narrow HOMO-LUMO gap. Moreover, the B—N unit also affects the aromaticity of the π-system as revealed by nucleus-independent chemical shift (NICS) via time-dependent density functional theory (TD-DFT) calculation. The single crystal structure analysis demonstrates that 1a has a helically twisted framework and Plus (P)/Minus (M) enantiomers. However, the Gibbs activation energy (ΔG≠(T)) of the enantiomerization at room temperature is too low to separate two enantiomers by chiral high performance liquid chromatography (HPLC). Furthermore, the B—N unit exhibits partial double bond character and the BN-containing six-membered ring shows weak aromaticity. 1a with a phenyl group exhibits the deboronization upon addition of trifluoroacetic acid (TFA) as well as a specific sensing behavior to fluoride ion. However, 1b shows no deboronization upon addition of TFA and no sensing behavior to fluoride ion due to its steric hindered mesityl (Mes) group, but has a reversible stimuli-responsiveness with acid and base, this proton-responsiveness is similar to all-carbon analogue 2.
Key words: azulene; BN-heteroaromatic; helicene; protonation
Chao Duan , Jianwei Zhang , Junjun Xiang , Xiaodi Yang , Xike Gao . Design, Synthesis and Properties of Azulene-Based BN-[4]Helicenes※[J]. Acta Chimica Sinica, 2022 , 80(1) : 29 -36 . DOI: 10.6023/A21110508
| [1] | (a) Clar, E. Polycyclic Hydrocarbons, Academic Press, London 1964. |
| [1] | (b) Anthony, J. E. Chem. Rev. 2006, 106, 5028. |
| [1] | (c) Wu, J.; Pisula, W.; Müllen, K. Chem. Rev. 2007, 107, 718. |
| [1] | (d) Wang, X.-Y.; Yao, X.; Müllen, K. Sci. China Chem. 2019, 62, 1099. |
| [2] | (a) Stępień, M.; Gońka, E.; Żyła, M.; Sprutta, N. Chem. Rev. 2017, 117, 3479. |
| [2] | (b) Hirai, M.; Tanaka, N.; Sakai, M.; Yamaguchi, S. Chem. Rev. 2019, 119, 8291. |
| [2] | (c) Lv, M.; Zhou, R.-M.; Lv, K.; Wei, Z.-X. Acta Chim. Sinica 2021, 79, 284. (in Chinese) |
| [2] | 吕敏, 周瑞敏, 吕琨, 魏志祥, 化学学报 2021, 79, 284). |
| [2] | (d) Li, L.-Y.; Zheng, W.; Li, C.-H. Acta Chim. Sinica 2021, 79, 81 ; (in Chinese) |
| [2] | 李凌燕, 郑玮, 李承辉, 化学学报 2021, 79, 81). |
| [2] | (e) Hu, X.-M.; Zhong, C.-X.; Li, X.-Y.; Jia, X.; Wei, Y.; Xie, L.-H. Acta Chim. Sinica 2021, 79, 953. (in Chinese) |
| [2] | 胡鑫明, 钟春晓, 李晓燕, 贾雄, 魏颖, 解令海, 化学学报 2021, 79, 953). |
| [3] | (a) Bosdet, M. J. D.; Piers, W. E. Can. J. Chem. 2009, 87, 8. |
| [3] | (b) Jaska, C. A.; Emslie, D. J. H.; Bosdet, M. J. D.; Piers, W. E.; Sorensen, T. S.; Parvez, M. J. Am. Chem. Soc. 2006, 128, 10885. |
| [3] | (c) Wang, X.-Y.; Zhuang, F.-D.; Wang, R.-B.; Wang, X.-C.; Cao, X.-Y.; Wang, J.-Y.; Pei, J. J. Am. Chem. Soc. 2014, 136, 3764. |
| [3] | (d) Dou, C.; Ding, Z.; Zhang, Z.; Xie, Z.; Liu, J.; Wang, L. Angew. Chem. Int. Ed. 2015, 54, 3648. |
| [3] | (e) Zhuang, F.-D.; Sun, Z.-H.; Yao, Z.-F.; Chen, Q.-R.; Huang, Z.; Yang, J.-H.; Wang, J.-Y.; Pei, J. Angew. Chem. Int. Ed. 2019, 58, 10708. |
| [3] | (f) Wang, X.; Zhang, F.; Schellhammer, K. S.; Machata, P.; Ortmann, F.; Cuniberti, G.; Fu, Y.; Hunger, J.; Tang, R.; Popov, A. A.; Berger, R.; Müllen, K.; Feng, X. J. Am. Chem. Soc. 2016, 138, 11606. |
| [3] | (g) Cao, Y.; Zhu, C.; Barlog, M.; Barker, K. P.; Ji, X.; Kalin, A. J.; Al-Hashimi, M.; Fang, L. J. Org. Chem. 2021, 86, 2100. |
| [3] | (h) Abengózar, A.; García-García, P.; Sucunza, D.; Pérez-Redondo, A.; Vaquero, J. J. Chem. Commun. 2018, 54, 2467. |
| [4] | (a) Wang, S.; Yang, D.-T.; Lu, J.; Shimogawa, H.; Gong, S.; Wang, X.; Mellerup, S. K.; Wakamiya, A.; Chang, Y.-L.; Yang, C.; Lu, Z.-H. Angew. Chem. Int. Ed. 2015, 54, 15074. |
| [4] | (b) Hashimoto, S.; Ikuta, T.; Shiren, K.; Nakatsuka, S.; Ni, J.; Nakamura, M.; Hatakeyama, T. Chem. Mater. 2014, 26, 6265. |
| [4] | (c) Qiang, P.; Sun, Z.; Wan, M.; Wang, X.; Thiruvengadam, P.; Bingi, C.; Wei, W.; Zhu, W.; Wu, D.; Zhang, F. Org. Lett. 2019, 21, 4575. |
| [5] | Crossley, D. L.; Cade, I. A.; Clark, E. R.; Escande, A.; Humphries, M. J.; King, S. M.; Vitorica-Yrezabal, I.; Ingleson, M. J.; Turner, M. L. Chem. Sci. 2015, 6, 5144. |
| [6] | Huang, H.; Zhou, Y.; Wang, M.; Zhang, J.; Cao, X.; Wang, S.; Cao, D.; Cui, C. Angew. Chem. Int. Ed. 2019, 58, 10132. |
| [7] | (a) Martin, R. H. Angew. Chem. Int. Ed. Engl. 1974, 13, 649. |
| [7] | (b) Shen, Y.; Chen, C.-F. Chem. Rev. 2012, 112, 1463. |
| [7] | (c) Gingras, M.; Felix, G.; Peresutti, R. Chem. Soc. Rev. 2013, 42, 1007. |
| [7] | (d) Gingras, M. Chem. Soc. Rev. 2013, 42, 1051. |
| [7] | (e) Gingras, M. Chem. Soc. Rev. 2013, 42, 968. |
| [8] | (a) Yamamoto, K.; Shimizu, T.; Igawa, K.; Tomooka, K.; Hirai, G.; Suemune, H.; Usui, K. Sci. Rep. 2016, 6, 36211. |
| [8] | (b) Nakamura, K.; Furumi, S.; Takeuchi, M.; Shibuya, T.; Tanaka, K. J. Am. Chem. Soc. 2014, 136, 5555. |
| [8] | (c) Isla, H.; Saleh, N.; Ou-Yang, J.-K.; Dhbaibi, K.; Jean, M.; Dziurka, M.; Favereau, L.; Vanthuyne, N.; Toupet, L.; Jamoussi, B.; Srebro-Hooper, M.; Crassous, J. J. Org. Chem. 2019, 84, 5383. |
| [8] | (d) Schulte, T. R.; Holstein, J. J.; Clever, G. H. Angew. Chem. Int. Ed. 2019, 58, 5562. |
| [8] | (e) Gicquel, M.; Zhang, Y.; Aillard, P.; Retailleau, P.; Voituriez, A.; Marinetti, A. Angew. Chem. Int. Ed. 2015, 54, 5470. |
| [8] | (f) Li, M.; Lin, W.-B.; Fang, L.; Chen, C.-F. Acta Chim. Sinica 2017, 75, 1150. (in Chinese) |
| [8] | 李猛, 林伟彬, 房蕾, 陈传峰, 化学学报 2017, 75, 1150). |
| [8] | (g) Chen, X.-Y.; Li, J.-K.; Wang, X.-Y. Chin. J. Org. Chem. 2021, 41, 4105. (in Chinese) |
| [8] | 陈星宇, 李继坤, 王小野, 有机化学 2021, 41, 4105). |
| [9] | Cahn, R. S.; Ingold, C.; Prelog, V. Angew. Chem. Int. Ed. Engl. 1966, 5, 385. |
| [10] | Martin, R. H.; Marchant, M. J. Tetrahedron 1974, 30, 347. |
| [11] | (a) Wheland, G. W.; Mann, D. E. J. Chem. Phys. 1949, 17, 264. |
| [11] | (b) Anderson, A. G.; Steckler, B. M. J. Am. Chem. Soc. 1959, 81, 4941. |
| [12] | Michl, J.; Thulstrup, E. W. Tetrahedron 1976, 32, 205. |
| [13] | Beer, M.; Longuet-Higgins, H. C. J. Chem. Phys. 1955, 23, 1390. |
| [14] | (a) Xin, H.; Li, J.; Yang, X.; Gao, X. J. Org. Chem. 2020, 85, 70. |
| [14] | (b) Pigulski, B.; Shoyama, K.; Würthner, F. Angew. Chem. Int. Ed. 2020, 59, 15908. |
| [14] | (c) Xin, H.; Li, J.; Lu, R.-Q.; Gao, X.; Swager, T. M. J. Am. Chem. Soc. 2020, 142, 13598. |
| [14] | (d) Sasaki, Y.; Takase, M.; Okujima, T.; Mori, S.; Uno, H. Org. Lett. 2019, 21, 1900. |
| [14] | (e) Smits, E. C. P.; Setayesh, S.; Anthopoulos, T. D.; Buechel, M.; Nijssen, W.; Coehoorn, R.; Blom, P. W. M.; de?Boer, B.; de?Leeuw, D. M. Adv. Mater. 2007, 19, 734. |
| [14] | (f) Yamaguchi, Y.; Maruya, Y.; Katagiri, H.; Nakayama, K.-i.; Ohba, Y. Org. Lett. 2012, 14, 2316. |
| [14] | (g) Yamaguchi, Y.; Ogawa, K.; Nakayama, K.-i.; Ohba, Y.; Katagiri, H. J. Am. Chem. Soc. 2013, 135, 19095. |
| [14] | (h) Yamaguchi, Y.; Takubo, M.; Ogawa, K.; Nakayama, K.-i.; Koganezawa, T.; Katagiri, H. J. Am. Chem. Soc. 2016, 138, 11335. |
| [14] | (i) Xin, H.; Ge, C.; Yang, X.; Gao, H.; Yang, X.; Gao, X. Chem. Sci. 2016, 7, 6701. |
| [14] | (j) Xin, H.; Ge, C.; Jiao, X.; Yang, X.; Rundel, K.; McNeill, C. R.; Gao, X. Angew. Chem. Int. Ed. 2018, 57, 1322. |
| [15] | (a) Zhu, C.; Shoyama, K.; Würthner, F. Angew. Chem. Int. Ed. 2020, 59, 21505. |
| [15] | (b) Zhang, X.-S.; Huang, Y.-Y.; Zhang, J.; Meng, W.; Peng, Q.; Kong, R.; Xiao, Z.; Liu, J.; Huang, M.; Yi, Y.; Chen, L.; Fan, Q.; Lin, G.; Liu, Z.; Zhang, G.; Jiang, L.; Zhang, D. Angew. Chem. Int. Ed. 2020, 59, 3529. |
| [15] | (c) Ogawa, N.; Yamaoka, Y.; Takikawa, H.; Yamada, K.-i.; Takasu, K. J. Am. Chem. Soc. 2020, 142, 13322. |
| [15] | (d) Konishi, A.; Horii, K.; Shiomi, D.; Sato, K.; Takui, T.; Yasuda, M. J. Am. Chem. Soc. 2019, 141, 10165. |
| [15] | (e) Liu, J.; Mishra, S.; Pignedoli, C. A.; Passerone, D.; Urgel, J. I.; Fabrizio, A.; Lohr, T. G.; Ma, J.; Komber, H.; Baumgarten, M.; Corminboeuf, C.; Berger, R.; Ruffieux, P.; Müllen, K.; Fasel, R.; Feng, X. J. Am. Chem. Soc. 2019, 141, 12011. |
| [15] | (f) Yang, X.; Rominger, F.; Mastalerz, M. Angew. Chem. Int. Ed. 2019, 58, 17577. |
| [15] | (g) Zhu, C.; Shoyama, K.; Würthner, F. Angew. Chem. Int. Ed. 2020, 59, 21505. |
| [15] | (h) Yamamoto, K. I. Y.; Tohnai, N.; Kakiuchi, F.; Aso, Y. Sci. Rep. 2018, 8, 17663. |
| [16] | (a) Uehara, K.; Mei, P.; Murayama, T.; Tani, F.; Hayashi, H.; Suzuki, M.; Aratani, N.; Yamada, H. Eur. J. Org. Chem. 2018, 4508. |
| [16] | (b) Yamamoto, K.; Okazumi, M.; Suemune, H.; Usui, K. Org. Lett. 2013, 15, 1806. |
| [16] | (c) Han, Y.; Xue, Z.; Li, G.; Gu, Y.; Ni, Y.; Dong, S.; Chi, C. Angew. Chem. Int. Ed. 2020, 59, 9026. |
| [16] | (d) Ma, J.; Fu, Y.; Dmitrieva, E.; Liu, F.; Komber, H.; Hennersdorf, F.; Popov, A. A.; Weigand, J. J.; Liu, J.; Feng, X. Angew. Chem. Int. Ed. 2020, 59, 5637. |
| [17] | Ortgies, S.; Breder, A. Org. Lett. 2015, 17, 2748. |
| [18] | Weimar, M.; Correa da Costa, R.; Lee, F.-H.; Fuchter, M. J. Org. Lett. 2013, 15, 1706. |
| [19] | Niu, W.; Ma, J.; Soltani, P.; Zheng, W.; Liu, F.; Popov, A. A.; Weigand, J. J.; Komber, H.; Poliani, E.; Casiraghi, C.; Droste, J.; Hansen, M. R.; Osella, S.; Beljonne, D.; Bonn, M.; Wang, H. I.; Feng, X.; Liu, J.; Mai, Y. J. Am. Chem. Soc. 2020, 142, 18293. |
| [20] | Fürstner, A.; Mamane, V. J. Org. Chem. 2002, 67, 6264. |
| [21] | Abbey, E. R.; Zakharov, L. N.; Liu, S.-Y. J. Am. Chem. Soc. 2008, 130, 7250. |
| [22] | (a) Chen, Y.; Chen, W.; Qiao, Y.; Lu, X.; Zhou, G. Angew. Chem. Int. Ed. 2020, 59, 7122. |
| [22] | (b) Zhuang, F. D.; Yang, J. H.; Sun, Z. H.; Zhang, P. F.; Chen, Q. R.; Wang, J. Y.; Pei, J. Chin. J. Chem. 2021, 39, 909. |
| [22] | (c) Campbell, P. G.; Marwitz, A. J. V.; Liu, S.-Y. Angew. Chem. Int. Ed. 2012, 51, 6074. |
| [22] | (d) Sun, Z.; Yi, C.; Liang, Q.; Bingi, C.; Zhu, W.; Qiang, P.; Wu, D.; Zhang, F. Org. Lett. 2020, 22, 209. |
| [22] | (e) Nakatsuka, S.; Yasuda, N.; Hatakeyama, T. J. Am. Chem. Soc. 2018, 140, 13562. |
| [22] | (f) Hatakeyama, T.; Hashimoto, S.; Oba, T.; Nakamura, M. J. Am. Chem. Soc. 2012, 134, 19600. |
| [22] | (g) Pati, P. B.; Jin, E.; Kim, Y.; Kim, Y.; Mun, J.; Kim, S. J.; Kang, S. J.; Choe, W.; Lee, G.; Shin, H.-J.; Park, Y. S. Angew. Chem. Int. Ed. 2020, 59, 14891. |
| [23] | Biet, T.; Fihey, A.; Cauchy, T.; Vanthuyne, N.; Roussel, C.; Crassous, J.; Avarvari, N. Chem. Eur. J. 2013, 19, 13160. |
| [24] | (a) Ravat, P. Chem. Eur. J. 2021, 27, 3957. |
| [24] | (b) Liu, B.-K.; Zhang, Y.-L.; Chen, Y.; Liu, X.-G.; Zhang, L. Chin. J. Org. Chem. 2020, 40, 2879. (in Chinese) |
| [24] | 刘秉康, 张艳丽, 陈瑜, 刘旭光, 张磊, 有机化学 2020, 40, 2879). |
| [25] | Xin, H.; Gao, X. ChemPlusChem 2017, 82, 945. |
| [26] | (a) Murai, M.; Iba, S.; Ota, H.; Takai, K. Org. Lett. 2017, 19, 5585. |
| [26] | (b) Hou, B.; Li, J.; Xin, H.-S.; Yang, X.-D.; Gao, H.-L.; Peng, P.-Z.; Gao, X.-K. Acta Chim. Sinica 2020, 78, 788. (in Chinese) |
| [26] | 侯斌, 李晶, 辛涵申, 杨笑迪, 高洪磊, 彭培珍, 高希珂, 化学学报 2020, 78, 788). |
| [27] | (a) Ju, C. W.; Li, B.; Li, L.; Yan, W.; Cui, C.; Ma, X.; Zhao, D. J. Am. Chem. Soc. 2021, 143, 5903. |
| [27] | (b) Xia, Y.; Zhang, M.; Ren, S.; Song, J.; Ye, J.; Humphrey, M. G.; Zheng, C.; Wang, K.; Zhang, X. Org. Lett. 2020, 22, 7942. |
| [27] | (c) Urban, M.; Durka, K.; Jankowski, P.; Serwatowski, J.; Lulinski, S. J. Org. Chem. 2017, 82, 8234. |
| [28] | Murfin, L. C.; Weber, M.; Park, S. J.; Kim, W. T.; Lopez-Alled, C. M.; McMullin, C. L.; Pradaux-Caggiano, F.; Lyall, C. L.; Kociok-Köhn, G.; Wenk, J.; Bull, S. D.; Yoon, J.; Kim, H. M.; James, T. D.; Lewis, S. E. J. Am. Chem. Soc. 2019, 141, 19389. |
| [29] | Brown, A. R.; Jarrett, C. P.; de Leeuw, D. M.; Matters, M. Synth. Met. 1997, 88, 37. |
| [30] | (a) Amir, E.; Amir, R. J.; Campos, L. M.; Hawker, C. J. J. Am. Chem. Soc. 2011, 133, 10046. |
| [30] | (b) Murai, M.; Amir, E.; Amir, R. J.; Hawker, C. J. Chem. Sci. 2012, 3, 2721. |
| [30] | (c) Amir, E.; Murai, M.; Amir, R. J.; Cowart, J. S.; Chabinyc, M. L.; Hawker, C. J. Chem. Sci. 2014, 5, 4483. |
| [30] | (d) Murai, M.; Ku, S.-Y.; Treat, N. D.; Robb, M. J.; Chabinyc, M. L.; Hawker, C. J. Chem. Sci. 2014, 5, 3753. |
| [30] | (e) Tsurui, K.; Murai, M.; Ku, S.-Y.; Hawker, C. J.; Robb, M. J. Adv. Funct. Mater. 2014, 24, 7338. |
| [30] | (f) Peng, P.-Z.; Li, J.; Hou, B.; Xin, H.-S.; Cheng, T.-Y.; Gao, X.-K. Chin. J. Org. Chem. 2020, 40, 3916. (in Chinese) |
| [30] | 彭培珍, 李晶, 侯斌, 辛涵申, 程探宇, 高希珂, 有机化学 2020, 40, 3916). |
| [31] | (a) Matsuo, K.; Saito, S.; Yamaguchi, S. J. Am. Chem. Soc. 2014, 136, 12580. |
| [31] | (b) Schickedanz, K.; Trageser, T.; Bolte, M.; Lerner, H.-W.; Wagner, M. Chem. Commun. 2015, 51, 15808. |
| [31] | (c) Miyamoto, F.; Nakatsuka, S.; Yamada, K.; Nakayama, K.-i.; Hatakeyama, T. Org. Lett. 2015, 17, 6158. |
| [31] | (d) Schickedanz, K.; Radtke, J.; Bolte, M.; Lerner, H.-W.; Wagner, M. J. Am. Chem. Soc. 2017, 139, 2842. |
| [31] | (e) Iida, A.; Yamaguchi, S. J. Am. Chem. Soc. 2011, 133, 6952. |
| [32] | Wade, C. R.; Broomsgrove, A. E. J.; Aldridge, S.; Gabbaï, F. P. Chem. Rev. 2010, 110, 3958. |
/
| 〈 |
|
〉 |