

Advances in Formation of C—X Bonds via Cleavage of C—N Bond of Quaternary Ammonium Salts
Received date: 2021-11-27
Online published: 2021-12-16
Supported by
National Natural Science Foundation of China(20672088); National Natural Science Foundation of China(21372034); Chengdu Science and Technology Bureau(2019-YF05-02395-SN); State Key Laboratory of Geohazard Prevention and Geoenvironment Protection(SKLGP2020Z003)
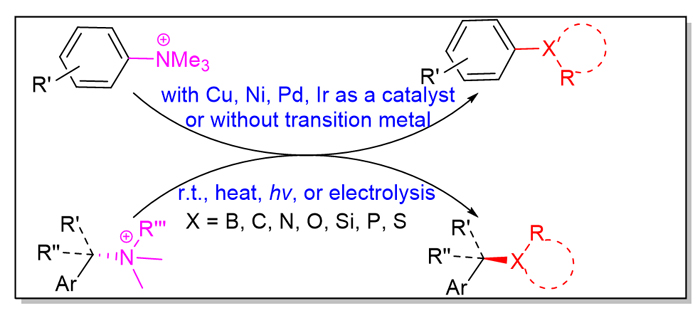
Amines have a wide variety and are readily available. Due to the larger C—N bond energy of amines, C—N bond of amines generally needs to be activated before cleavage. In recent years, a variety of activation methods of amino groups have been developed. Among the activation methods, converting amines into quaternary ammonium salts has certain advantages of easy preparation and stable storage. In last decade, quaternary ammonium salts derived from aromatic amines and benzylamines have made great progress in the study of constructing various C—X bonds via cleavage of C—N bond. This review mainly discusses the formation of C—X bonds of quaternary ammonium salts via cleavage of C—N bond with or without transition metal catalysts over the past few years. Via C—N bond breakage, quaternary ammonium salts can construct C—B bond, C—C bond, C—N bond, C—O bond, C—Si bond, C—P bond, C—S bond, C—Se bond, and so on. And thus borates, aromatics, alkanes, ethers, amines, silanes, phosphines, thioethers, disulfides, selenoethers, diselenides and other compounds are synthesized. Moreover, if the quaternary ammonium salts derived from chiral benzylamines are adopted, a variety of highly enantiopure chiral organic compounds are obtained. The chiralities of quaternary ammonium salts are excellently kept in the products, and SN2 type configuration reversal occurs for all of the reported reactions.
Yiding Wang , Fuhai Li , Qingle Zeng . Advances in Formation of C—X Bonds via Cleavage of C—N Bond of Quaternary Ammonium Salts[J]. Acta Chimica Sinica, 2022 , 80(3) : 386 -394 . DOI: 10.6023/A21110536
| [1] | Hartwig, J. F. Acc. Chem. Res. 1998, 31, 852. |
| [2] | Ley, S. V.; Thomas, A. W. Angew. Chem. Int. Ed. 2003, 42, 5400. |
| [3] | Schlummer, B.; Scholz, U. Adv. Synth. Catal. 2004, 346, 1599. |
| [4] | Ouyang, K.; Hao, W.; Zhang, W. X.; Xi, Z. Chem. Rev. 2015, 115, 12045. |
| [5] | Wang, Q.; Su, Y.; Li, L.; Huang, H. Chem. Soc. Rev. 2016, 45, 1257. |
| [6] | Wang, Z. X.; Yang, B. Org. Biomol. Chem. 2020, 18, 1057. |
| [7] | Li, G.; Chen, Y.; Xia, J. B. Chin. J. Org. Chem. 2018, 38, 1949. (in Chinese) |
| [7] | (李刚, 陈烨, 夏纪宝, 有机化学, 2018, 38, 1949.) |
| [8] | Song, M. M.; Zhang, Z. G.; Zheng, D.; Li, X.; Liang, R.; Zhao, X. N.; Shi, L.; Zhang, G. S. Chin. J. Org. Chem. 2020, 40, 2433. (in Chinese) |
| [8] | (宋蒙蒙, 张志国, 郑丹, 李祥, 梁蕊, 赵旭娜, 时蕾, 张贵生, 有机化学, 2020, 40, 2433.) |
| [9] | Menggen, Q.; Wu, Y.; Bao, Y. S. Chin. J. Org. Chem. 2018, 38, 902. (in Chinese) |
| [9] | (孟根其其格, 乌云, 包永胜, 有机化学, 2018, 38, 902.) |
| [10] | Zhao, Y.; Li, S. H.; Zhang, M. M.; Liu, F. Acta Chim. Sinica 2019, 77, 916. (in Chinese) |
| [10] | (赵勇, 李施宏, 张苗苗, 刘峰, 化学学报, 2019, 77, 916.) |
| [11] | Liu, J.; Yang, Y.; Ouyang, K.; Zhang, W. X. Green Synth. Catal. 2021, 2, 87. |
| [12] | Wang, C. Chem. Pharm. Bull. 2020, 68, 683. |
| [13] | Bao, H.; Qi, X.; Tambar, U. K. J. Am. Chem. Soc. 2011, 133, 1206. |
| [14] | Wenkert, E.; Han, A. L.; Jenny, C. J. J. Chem. Soc. Chem. Commnu. 1988, 975. |
| [15] | Reeves, J. T.; Fandrick, D. R.; Tan, Z.; Song, J. J.; Lee, H.; Yee, N. K.; Senanayake, C. H. Org. Lett. 2010, 12, 4388. |
| [16] | Guo, W. J.; Wang, Z. X. Tetrahedron 2013, 69, 9580. |
| [17] | Xie, L. G.; Wang, Z. X. Angew. Chem. Int. Ed. 2011, 50, 4901. |
| [18] | Ogawa, H.; Yang, Z. K.; Minami, H.; Kojima, K.; Saito, T.; Wang, C.; Uchiyama, M. ACS Catal. 2017, 7, 3988. |
| [19] | Wang, D. Y.; Kawahata, M.; Yang, Z. K.; Miyamoto, K.; Komagawa, S.; Yamaguchi, K.; Wang, C.; Uchiyama, M. Nature Commun. 2016, 7, 12937. |
| [20] | Yang, Z. K.; Wang, D. Y.; Minami, H.; Ogawa, H.; Ozaki, T.; Saito, T.; Miyamoto, K.; Wang, C.; Uchiyama, M. Chem. Eur. J. 2016, 22, 15693. |
| [21] | Blakey, S. B.; MacMillan, D. W. C. J. Am. Chem. Soc. 2003, 125, 6046. |
| [22] | Maity, P.; Shacklady-McAtee, D. M.; Yap, G. P. A.; Sirianni, E. R.; Watson, M. P. J. Am. Chem. Soc. 2013, 135, 280. |
| [23] | Chen, Q.; Gao, F.; Tang, H.; Yao, M.; Zhao, Q.; Shi, Y.; Dang, Y.; Cao, C. ACS Catal. 2019, 9, 3730. |
| [24] | Xu, S.; Zhang, Z.; Han, C.; Hu, W.; Xiao, T.; Yuan, Y.; Zhao, J. J. Org. Chem. 2019, 84, 12192. |
| [25] | Zhu, F.; Tao, J. L.; Wang, Z. X. Org. Lett. 2015, 17, 4926. |
| [26] | Han, C.; Zhang, Z.; Xu, S.; Wang, K.; Chen, K.; Zhao, J. J. Org. Chem. 2019, 84, 16308. |
| [27] | Moragas, T.; Gaydou, M.; Martin, R. Angew. Chem. Int. Ed. 2016, 55, 5053. |
| [28] | Liao, L. L.; Cao, G. M.; Ye, J. H.; Sun, G. Q.; Zhou, W. J.; Gui, Y. Y.; Yan, S. S.; Shen, G.; Yu, D. G. J. Am. Chem. Soc. 2018, 140, 17338. |
| [29] | Yu, W.; Yang, S.; Xiong, F.; Fan, T.; Feng, Y.; Huang, Y.; Fu, J.; Wang, T. Org. Biomol. Chem. 2018, 16, 3099. |
| [30] | Rand Alexander, W.; Montgomery, J. Chem. Sci. 2019, 10, 5338. |
| [31] | Scharfbier, J.; Gross, B. M.; Oestreich, M. Angew. Chem. Int. Ed. 2020, 59, 1577. |
| [32] | Zhang, X. Q.; Wang, Z. X. Org. Biomol. Chem. 2014, 12, 1448. |
| [33] | Chen, H.; Yang, H.; Li, N.; Xue, X.; He, Z.; Zeng, Q. Org. Proc. Res. Dev. 2019, 23, 1679. |
| [34] | Yang, B.; Wang, Z. X. J. Org. Chem. 2019, 84, 1500. |
| [35] | Li, N.; Chen, F.; Wang, G.; Zeng, Q. Monatsch. Chem. 2020, 151, 99. |
| [36] | O'Connor, S. E.; Grosset, A.; Janiak, P. Fund. Clin. Pharmacol. 1999, 13, 145. |
| [37] | Sobal, G.; Menzel, E. J.; Sinzinger, H. Biochem. Pharmacol. 2001, 61, 373. |
| [38] | Zeng, Q.; Wang, H.; Wang, T.; Cai, Y.; Weng, W.; Zhao, Y. Adv. Synth. Catal. 2005, 347, 1933. |
| [39] | Zhang, L.; Tan, M.; Zhou, L.; Zeng, Q. Tetrahedron Lett. 2018, 59, 2778. |
| [40] | Jiang, W.; Huang, Y.; Zhou, L.; Zeng, Q. Sci. China Chem. 2019, 62, 1213. |
| [41] | Feng, J.; Zhang, Q.; Li, F.; Yang, L.; Kuchukulla, R. R.; Zeng, Q. Synlett 2021, 32, 224. |
| [42] | Jiang, W.; Li, N.; Zhou, L.; Zeng, Q. ACS Catal. 2018, 8, 9899. |
| [43] | Chen, H.; Jiang, W.; Zeng, Q. Chem. Rec. 2020, 20, 1269. |
| [44] | Chen, H.; Zhang, Q.; Zheng, W.; Yang, H.; Zeng, Q. Asian J. Org. Chem. 2020, 9, 773. |
| [45] | Huang, Y.; Chen, H.; Zheng, W.; Zeng, Q. Tetrahedron Lett. 2020, 61, 152320. |
| [46] | Huang, Y.; Li, J.; Chen, H.; He, Z.; Zeng, Q. Chem. Rec. 2021, 21, 1216. |
| [47] | Zhang, H.; Hagihara, S.; Itami, K. Chem. Eur. J. 2015, 21, 16796. |
| [48] | Hu, J.; Sun, H.; Cai, W.; Pu, X.; Zhang, Y.; Shi, Z. J. Org. Chem. 2016, 81, 14. |
| [49] | Basch, C. H.; Cobb, K. M.; Watson, M. P. Org. Lett. 2016, 18, 136. |
| [50] | Gui, Y.; Tian, S. K. Org. Lett. 2017, 19, 1554. |
| [51] | Li, F.; Wang, D.; Chen, H.; He, Z.; Zhou, L.; Zeng, Q. Chem. Commun. 2020, 56, 13029. |
| [52] | Tang, Q.; Li, F.; Chen, F.; Yin, X.; Tang, Y.; Zeng, Q. Asian J. Org. Chem. 2021, 10, 1687. |
| [53] | Chen, F.; Li, F.; Zeng, Q. Eur. J. Org. Chem. 2021, 2021, 5605. |
| [54] | Zhang, Q.; Feng, H.; Yang, H.; He, Z.; Zeng, Q. J. Org. Chem. 2021, 86, 7806. |
| [55] | Yang, L.; Wang, B.; Yin, X.; Zeng, Q. Chem. Rec. 2021, DOI: 10.1002/tcr.202100242. |
| [56] | Mfuh, A. M.; Doyle, J. D.; Chhetri, B.; Arman, H. D.; Larionov, O. V. J. Am. Chem. Soc. 2016, 138, 2985. |
| [57] | Wang, D. Y.; Yang, Z. K.; Wang, C.; Zhang, A.; Uchiyama, M. Angew. Chem. Int. Ed. 2018, 57, 3641. |
| [58] | Wang, D. Y.; Wen, X.; Xiong, C. D.; Zhao, J. N.; Ding, C. Y.; Meng, Q.; Zhou, H.; Wang, C.; Uchiyama, M.; Lu, X. J.; Zhang, A. iScience 2019, 15, 307. |
| [59] | Yang, D. T.; Zhu, M.; Schiffer, Z. J.; Williams, K.; Song, X.; Liu, X.; Manthiram, K. ACS Catal. 2019, 9, 4699. |
/
| 〈 |
|
〉 |